相与形貌控制MnO2的合成及其对尖晶石LiMn2O4正极材料电化学性能的影响
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
本研究以KMnO4、K2S2O8、KClO3、(NH4)2S2O8四种不同的氧化剂为原料,采用水热法合成了MnO2。KMnO4前驱体导致α- mno2聚集形成,而K2S2O8则产生α-和γ-MnO2的混合相。(NH4)2S2O8在较低温度下促进γ-MnO2的形成,在较高温度下诱导结构转变为β-MnO2。在所研究的锂前驱体中,LiOH被发现在合成过程中最有效地保持了LiMn2O4的球形形态。电化学测量结果表明,γ-MnO2合成的LiMn2O4样品的充电容量最高,为132.59 mAh∙g−1,而α- mno2基LiMn2O4则表现出最好的稳定性。这些结果表明,初始MnO2相对尖晶石阴极的电化学性能有显著影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
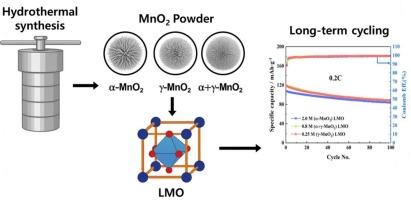
Phase- and morphology-controlled MnO2: Its synthesis and influence on the electrochemical performance of spinel LiMn2O4 cathode materials
In this study, MnO2 was synthesized via a hydrothermal method using four different oxidizing agents: KMnO4, K2S2O8, KClO3, and (NH4)2S2O8. The KMnO4 precursor led to the formation of aggregated α-MnO₂, while K2S2O8 produced a mixed phase of α- and γ-MnO2. (NH4)2S2O8 promoted the formation of γ-MnO2 at lower temperatures and induced a structural transition to β-MnO2 at elevated temperatures. Among the lithium precursors investigated, LiOH was found to be the most effective in preserving the spherical morphology of LiMn2O4 during synthesis. Electrochemical measurements revealed that the LiMn2O4 sample synthesized from γ-MnO2 exhibited the highest charge capacity of 132.59 mAh∙g−1, while the α-MnO2-based LiMn2O4 demonstrated the best stability. These results indicate that the initial MnO2 phase significantly influences the electrochemical performance of the resulting spinel cathode.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


