远端细胞通过突触样接触将必需的wnt直接传递给小鼠肠干细胞
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
Wnt传递到肠道干细胞生态位的时空控制调节肠道内稳态。远端细胞是特化的基质细胞,具有特征性的长而薄的细胞质突起,为这个生态位的发育和维持产生必要的wnt。然而,wnt如何在肠道中从远端细胞转移到干细胞尚不清楚。与肠道类器官共培养的小鼠远端细胞的荧光和电子显微镜发现了特化的远端细胞延伸,它们在微囊上运输和局部分泌wnt,并与上皮细胞密切接触,使人想起基于神经元接触的信号传导。在研究突触形成和质膜相关平台蛋白的潜在作用时,我们发现,远端细胞中KANK1或Liprins的缺失显著减少了它们的丝足,损害了WNT2向上皮细胞的呈递,并损害了远端细胞依赖的类器官生长。特异的远端细胞结构促进Wnt通过突触样接触传递到肠道干细胞壁龛。本文章由计算机程序翻译,如有差异,请以英文原文为准。
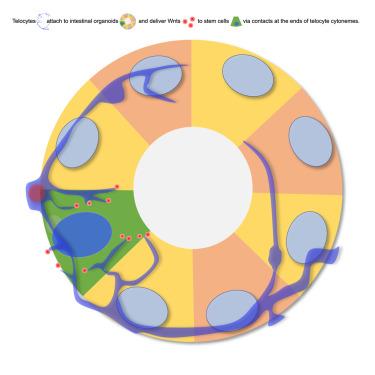
Telocytes deliver essential Wnts directly to murine intestinal stem cells via synapse-like contacts
Spatial and temporal control of Wnt delivery to the intestinal stem cell niche regulates intestinal homeostasis. Telocytes, specialized stromal cells with characteristic long, thin cytoplasmic protrusions, produce essential Wnts for the development and maintenance of this niche. However, how Wnts travel from telocytes to stem cells in the gut remains unclear. Fluorescence and electron microscopy of murine telocytes co-cultured with intestinal organoids identified specialized telocyte extensions that transport and locally secrete Wnts on microvesicles and make intimate contacts with epithelial cells, reminiscent of neuronal contact-based signaling. Investigating the potential role of synapse-forming and plasma membrane-associated platform proteins, we found that depletion of either KANK1 or Liprins from telocytes markedly reduced their filopodia, compromised WNT2 presentation to epithelial cells, and impaired telocyte-dependent organoid growth. Characteristic telocyte structures facilitate Wnt delivery to the intestinal stem cell niche via synapse-like contacts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


