外消旋叠氮嘧啶的不对称开环转化:苯二氮卓类药物的对映选择性途径。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了一个动态动力学分解(DKR)和动态动力学不对称转化(DyKAT)的例子,特别是在外消旋2-芳基-n - tosyziridine的开环转化中,采用战略性设计的n -亲核试剂和Cu(I)-(S)- binap作为手性Lewis酸催化剂,提供了理想的开环产物,收率(高达98%)和优异的对端选择性(高达99% ee)。用AgNO3/DBU进行分子内环化的开环产物以优异的收率(高达88%)和对映选择性(高达97% ee)制备了各种1,4-苯二氮卓类衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
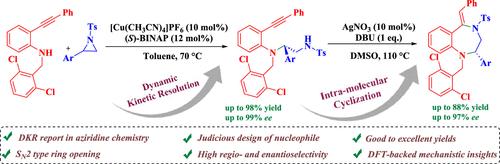
Asymmetric Ring-Opening Transformation of Racemic Aziridines: Enantioselective Route to Benzodiazepines
We report an example of dynamic kinetic resolution (DKR) in general and dynamic kinetic asymmetric transformation (DyKAT) in particular in the ring-opening transformation of racemic 2-aryl-N-tosylaziridines employing a strategically designed N-nucleophile and Cu(I)-(S)-BINAP as the chiral Lewis acid catalyst, furnishing the desired ring-opening products with excellent yield (up to 98%) and excellent enantioselectivity (up to 99% ee). The ring-opening products on intramolecular cyclization using AgNO3/DBU produced various 1,4-benzodiazepine derivatives in excellent yields (up to 88%) and enantioselectivity (up to 97% ee).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


