在果蝇达到临界体重时,Cullin1调控胰岛素/mTOR信号传导,驱动前胸腺内循环进程和表皮甾体生成。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
临界体重(CW)是昆虫发育的一个关键阈值,超过这个阈值,幼虫就开始向蛹转化。前胸腺(PG),负责产生外甾体,在控制这种转变的时间方面起着至关重要的作用。营养依赖的内循环是一种经过修饰的细胞周期,它忽略了有丝分裂,协调PG的大小和活性,从而影响CW获得的时间。然而,营养信号如何决定PG细胞内循环过程的分子机制仍未完全揭示。在这项研究中,我们发现了一个保守的SCF (SkpA-Cullin1-Slmb) E3连接酶复合物,在调节果蝇PG细胞的内循环事件中起关键作用。cullin1 (cul1)是该复合物的核心成分,其功能破坏可引起内周期抑制,减少体外甾体激素的生物合成和发育停滞。这种表型可以通过过度表达细胞周期蛋白E来挽救,细胞周期蛋白E可以在类固醇组织中诱导轮内环。值得注意的是,Cul1在CW检查点高表达。CW期前的饥饿可以抑制其表达。因此,胰岛素或雷帕霉素(TOR)信号靶点的缺失可以显著降低Cul1信号,表明该基因是一个营养应答基因。综上所述,我们的数据显示Cul1可能作为胰岛素/mTOR信号通路的下游调节因子,决定PG细胞中CW周围的内环过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
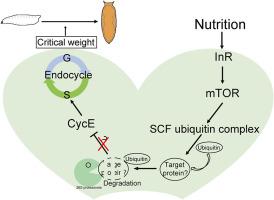
Cullin1 orchestrates insulin/mTOR signaling to drive endocycle progression and ecdysteroid production in Drosophila prothoracic glands during critical weight attainment
Critical weight (CW) is a key developmental threshold in insects, beyond which larvae initiate the transformation into pupae. The prothoracic gland (PG), responsible for producing ecdysteroids, plays a crucial role in controlling the timing of this transition. The nutrition dependent endocycle, a modified cell cycle that omits mitosis, coordinates the PG size and activity to influence the timing of CW attainment. However, the molecular mechanisms underlying how nutrient signals determine the endocycle process in PG cells are still not fully uncovered. In this study, we found a conserved SCF (SkpA-Cullin1-Slmb) E3 ligase complex that plays a critical role in regulating endocycle events in Drosophila melanogaster PG cells. Functional disruption of cullin1 (cul1), a core component of this complex, could cause endocycle inhibition, decrease the biosynthesis of ecdysteriod and developmental arrest. The phenotype can be rescued by overexpression cyclin E which may induce rounds of endocycles in the steroidogenic tissue. Remarkably, Cul1 was highly expressed during the CW checkpoint. Starvation before the CW period could repress its expression. In line with this, loss of insulin or target of rapamycin (TOR) signaling could significantly decrease the Cul1 signal during around CW indicating the gene is a nutrient responsive gene. Taken together, our data revealed that Cul1 could serve as downstream regulator of insulin/mTOR signaling pathway to determine the endocycling process around CW in PG cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


