可见光诱导二芳基烯丙醇中1,2-芳基自由基向1,5-二羰基化合物的迁移
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在无添加剂的条件下,Ir(III)光催化、可见光驱动的α,α-二芳烯丙基醇烷基酯化反应产生具有广泛官能团耐受性的1,5-二羰基。与富电子/邻位取代类似物相比,缺电子芳基表现出更高的迁移效率,机制研究暗示烷基酯自由基中间体。这种可持续的方法在温和的条件下运行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
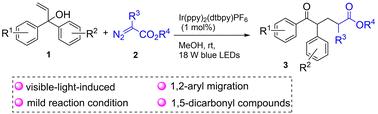
Visible-light-induced radical 1,2-aryl migration in diaryl allyl alcohols for efficient synthesis of 1,5-dicarbonyl compounds†
Herein, we developed a protocol for Ir(iii)-photocatalyzed, visible-light-driven radical 1,2-aryl migration in diaryl allyl alcohols, leading to the formation of 1,5-dicarbonyl compounds under additive-free conditions, with broad substrate tolerance. Electron-deficient aryl groups exhibit enhanced migration efficiency compared to electron-rich or ortho-substituted analogs. Mechanistic studies implicated the formation of alkyl ester radical intermediates. This is a sustainable method that can be performed under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


