大麻二酚改善左旋多巴诱导的运动障碍,调节神经炎症和内源性大麻素、内源性香草素和氮能系统。
IF 3.9
2区 医学
Q1 CLINICAL NEUROLOGY
Progress in Neuro-Psychopharmacology & Biological Psychiatry
Pub Date : 2025-07-18
DOI:10.1016/j.pnpbp.2025.111456
引用次数: 0
摘要
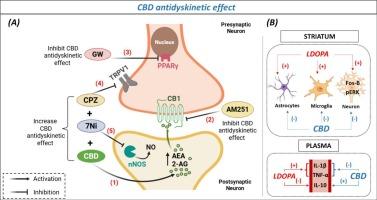
尽管l -3,4-二羟基苯丙氨酸(L-DOPA)作为帕金森病(PD)多巴胺(DA)替代的金标准被广泛使用,但其长期使用经常导致L-DOPA诱导的运动障碍(LID),这是一个重大的治疗挑战。调节内源性大麻素系统已成为一种有前途的方法来管理LID。本研究探讨大麻二酚(CBD),大麻二酚的一种非精神活性化合物,和PECS-101, CBD的氟化衍生物,是否可以减轻LID的发生和进展。我们使用单侧6-羟多巴胺损伤大鼠,用左旋多巴(10 mg kg - 1)治疗3周,诱导严重异常不自主运动(AIMs)。在最后两周进行治疗。CBD(30 mg kg - 1)和PECS-101(3和30 mg kg - 1)显著降低AIMs,而不损害左旋多巴的运动益处。CBD的抗运动障碍作用与纹状体Fos-B和phospho-ERK表达降低有关,与病变严重程度无关。CB1(1 mg kg - 1)和PPARγ(4 mg kg - 1)受体拮抗剂可阻止CBD的作用。与TRPV-1拮抗剂capsazepine(5 mg kg - 1)联合使用可增强CBD的抗运动障碍作用。辣椒素与神经元型一氧化氮合酶抑制剂7-硝基咪唑(10 mg kg - 1)联合使用可增强上述效果。CBD没有改变纹状体DA水平,但显著增加了运动障碍动物的anandamide和2-花生四烯醇甘油的浓度。CBD的抗运动障碍作用与纹状胶质细胞和外周炎症标志物的减少有关。这些发现表明,CBD通过与氮能神经传递和TRPV-1、CB1和PPARγ受体相互作用来缓解LID。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cannabidiol improves L-DOPA-induced dyskinesia and modulates neuroinflammation and the endocannabinoid, endovanilloid and nitrergic systems
Despite the widespread use of L-3,4-dihydroxyphenylalanine (L-DOPA) as the gold standard for dopamine (DA) replacement in Parkinson's Disease (PD), its prolonged administration frequently leads to L-DOPA-induced dyskinesia (LID), a significant therapeutic challenge. Modulating the endocannabinoid system has emerged as a promising approach for managing LID. This study explored whether cannabidiol (CBD), a non-psychoactive compound of Cannabis sativa, and PECS-101, a fluorinated derivative of CBD, could mitigate the onset and progression of LID. We used unilateral 6-hydroxydopamine-lesioned rats, treated with L-DOPA (10 mg kg − 1) for three weeks to induce severe abnormal involuntary movements (AIMs). Treatments were administered during the final two weeks. CBD (30 mg kg − 1) and PECS-101 (3 and 30 mg kg − 1) significantly reduced AIMs without impairing the motor benefits of L-DOPA. The antidyskinetic effects of CBD were associated with decreased striatal Fos-B and phospho-ERK expression and were independent of lesion severity. CBD effects were prevented by antagonists of CB1 (1 mg kg − 1) and PPARγ (4 mg kg − 1) receptors. Co-administration of TRPV-1 antagonist capsazepine (5 mg kg − 1) enhanced the antidyskinetic effects of CBD. Combining the capsazepine with the neuronal nitric oxide synthase inhibitor, 7-nitroimidazole (10 mg kg − 1) enhanced these effects. CBD did not alter striatal DA levels but significantly increased the concentrations of anandamide and 2-arachidonoylglycerol in dyskinetic animals. The antidyskinetic effects of CBD were associated with a reduction of the enhanced striatal glia and peripheral inflammation markers. These findings suggest that CBD alleviates LID by interacting with the nitrergic neurotransmission and TRPV-1, CB1, and PPARγ receptors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.00
自引率
1.80%
发文量
153
审稿时长
56 days
期刊介绍:
Progress in Neuro-Psychopharmacology & Biological Psychiatry is an international and multidisciplinary journal which aims to ensure the rapid publication of authoritative reviews and research papers dealing with experimental and clinical aspects of neuro-psychopharmacology and biological psychiatry. Issues of the journal are regularly devoted wholly in or in part to a topical subject.
Progress in Neuro-Psychopharmacology & Biological Psychiatry does not publish work on the actions of biological extracts unless the pharmacological active molecular substrate and/or specific receptor binding properties of the extract compounds are elucidated.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


