具有增强和稳定产氢活性的CuO@Cu7S4立方光催化剂的相变
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-07-21
DOI:10.1016/j.jtice.2025.106297
引用次数: 0
摘要
与其他半导体形成异质结构可以阻碍电子-空穴复合,提高光催化活性。CuO@Cu7S4纳米材料尚未用于光催化制氢应用。方法合成立方氧化亚铜,然后用硫化钠离子交换法制备CuO@Cu7S4核壳光催化剂。随着Na2S浓度的增加,硫化过程中发生了一系列的相变,依次形成Cu2O、Cu2O@Cu7S4、Cu2O/CuO@Cu7S4和CuO@Cu7S4光催化剂。硫化后Cu7S4壳层的形成也改变了表面形貌。Tauc图分析、紫外光电子能谱(UPS)和电子顺磁共振(EPR)测量表明CuO@Cu7S4光催化剂具有s型能带结构。CuO@Cu7S4复合光催化剂具有强光吸收、低电荷转移阻力、高效的电子空穴分离和增强的产氢活性。CuO@Cu7S4异质结光催化性能的提高是由于Cu7S4通过硫化在原位生长,促进了CuO和Cu7S4之间的紧密界面接触。优化后的CuO@Cu7S4光催化剂(COCS3)产氢活性达到11,934 μmol g-1h-1。经过3次循环后,仍保持90.4%的产氢活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
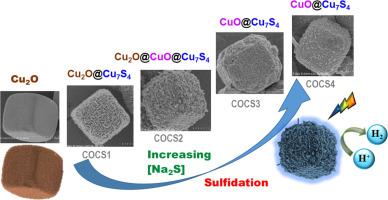
Phase change of core-shell CuO@Cu7S4 cubic photocatalysts with enhanced and stable H2 production activity
Background
Forming heterostructures of CuO with other semiconductors can hinder the electron-hole recombination and enhance photocatalytic activity. CuO@Cu7S4 nanomaterials have not been used for the photocatalytic H2 generation application.
Methods
The cubic cuprous oxide was synthesized, and then sulfidation by an ion exchange method using sodium sulfide to prepare CuO@Cu7S4 core-shell photocatalyst.
Significant findings
During the sulfidation process with increasing concentrations of Na2S, a series of phase transitions occur, sequentially forming the photocatalysts Cu2O, Cu2O@Cu7S4, Cu2O/CuO@Cu7S4, and CuO@Cu7S4. The surface morphology also changes as the Cu7S4 shell forms after sulfidation. Tauc plot analysis, ultraviolet photoelectron spectroscopy (UPS), and electron paramagnetic resonance (EPR) measurements indicate that the CuO@Cu7S4 photocatalyst possesses an S-scheme band structure. The CuO@Cu7S4 composite photocatalyst demonstrates strong light absorption, low charge transfer resistance, efficient electron-hole separation, and enhanced hydrogen (H2) production activity. The improved photocatalytic performance of the CuO@Cu7S4 heterojunction is attributed to the in-situ growth of Cu7S4 via sulfidation, which facilitates intimate interfacial contact between CuO and Cu7S4. The H2 production activity of optimized CuO@Cu7S4 photocatalyst (COCS3) reaches 11,934 μmol g-1h-1. 90.4 % of hydrogen production activity was retained after three repeated cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


