钯催化硝基芳烃与丁-1,3-二烯的反硝化Mizoroki-Heck反应。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了钯催化硝基芳烃与丁-1,3-二烯的反硝化Mizoroki-Heck反应,实现了1,4-二芳基-1,3-丁二烯的高效合成,具有较高的官能团耐受性和中优产率。在PhCF3中CsF存在的情况下,Pd(acac)2/BrettPhos作为催化剂的优化条件有利于选择性末端C-C偶联。底物范围包括多种硝基芳烃(富电子、贫电子、杂环)和二烯,具有广泛的适用性。产品的后功能化进一步强调了该方法在构建复杂芳基取代二烯中的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
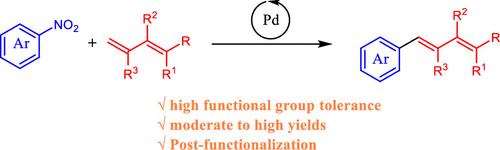
Palladium-Catalyzed Denitrative Mizoroki–Heck Reaction of Nitroarenes and Buta-1,3-dienes
A palladium-catalyzed denitrative Mizoroki–Heck reaction between nitroarenes and buta-1,3-dienes has been developed, enabling efficient synthesis of 1,4-diaryl-1,3-butadienes with a high functional group tolerance and moderate to excellent yields. Optimized conditions employing Pd(acac)2/BrettPhos as the catalyst in the presence of CsF in PhCF3 facilitate selective terminal C–C coupling. The substrate scope encompasses diverse nitroarenes (electron-rich, -poor, heterocyclic) and dienes, demonstrating broad applicability. Postfunctionalization of products further underscores the method’s utility in constructing complex aryl-substituted dienes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


