干细胞治疗对脊髓损伤患者运动恢复和神经性疼痛的缓解:综述和荟萃分析。
IF 2.2
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
研究设计:概括性回顾和荟萃分析。目的:本综述和荟萃分析旨在评估干细胞治疗对脊髓损伤(SCI)啮齿动物运动恢复和神经性疼痛缓解的疗效。方法:在Medline, Embase, Scopus和Web of Science中进行全面的文献检索,直到2024年5月,以确定干细胞治疗SCI的系统综述/荟萃分析。根据预先确定的纳入标准对这些综述中的原始研究进行筛选。提取运动、热痛觉过敏和机械异常性痛的数据。计算95%置信区间(ci)的标准化平均差(SMD)并汇总以确定总体效应大小。进行亚组分析和元回归,以研究每种干细胞类型的最佳疗效条件。结果:纳入31项系统综述/荟萃分析,涉及323项原始研究(516项实验,11,290只啮齿动物)。不同类型的干细胞均可显著恢复运动能力,其中脐带来源的间充质干细胞(U-MSCs) (SMD = 2.34, 95% CI 1.76-2.93)和少突胶质细胞祖细胞(OPCs) (SMD = 2.14, 95% CI 1.24-3.03)表现出较好的疗效。只有骨髓间充质干细胞(BM-MSCs)能缓解机械异常性痛(SMD = 1.33, 95% CI 0.61-2.05)。亚组分析显示,干细胞治疗的疗效取决于损伤模型、损伤治疗间隔、干细胞剂量和抗生素/免疫抑制剂的使用。证据评估的确定性显示,U-MSC在运动恢复方面的确定性为高,BM-MSC在缓解疼痛方面的确定性为中等,OPCs在运动恢复方面的确定性为低。结论:在中等至高的确定性下,我们的研究证明了间充质干细胞,特别是U-MSCs,在损伤后立即给予低剂量无抗生素的运动恢复和BM-MSCs缓解疼痛的卓越功效。这些发现提示在最佳条件下对这些干细胞类型进行进一步的临床研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
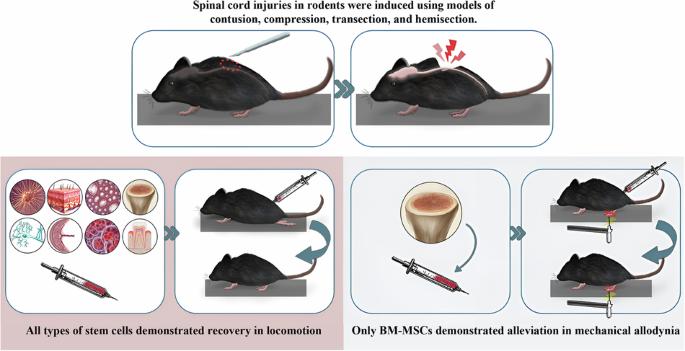
Stem cell therapy for locomotion recovery and neuropathic pain alleviation in spinal cord injury: an umbrella review and meta-analysis
An Umbrella Review and Meta-analysis. This umbrella review and meta-analysis aims to evaluate the efficacy of stem cell therapy for locomotion recovery and neuropathic pain alleviation in rodent models of spinal cord injury (SCI). A comprehensive literature search was conducted in Medline, Embase, Scopus, and Web of Science until May 2024 to identify systematic reviews/meta-analyses on stem cell therapy for SCI. Original studies from these reviews were screened based on the predefined inclusion criteria. Data on locomotion, thermal hyperalgesia, and mechanical allodynia were extracted. Standardized mean differences (SMD) with 95% confidence intervals (CIs) were calculated and pooled to determine overall effect sizes. Subgroup analyses and meta-regressions were performed to investigate the optimal conditions for efficacy in each stem cell type. 31 systematic reviews/meta-analyses with 323 original studies (516 experiments, 11,290 rodents) were included. Significant locomotion recovery was observed across stem cell types, with umbilical cord-derived mesenchymal stem cells (U-MSCs) (SMD = 2.34, 95% CI 1.76–2.93) and oligodendrocyte progenitor cells (OPCs) (SMD = 2.14, 95% CI 1.24–3.03) demonstrating superior efficacy. Only bone marrow-derived mesenchymal stem cells (BM-MSCs) alleviated mechanical allodynia (SMD = 1.33, 95% CI 0.61–2.05). Subgroup analysis showed that the efficacy of stem cell therapy is dependent on injury models, injury to treatment interval, stem cell dosage, and use of antibiotics/immunosuppressants. The certainty of evidence assessment showed high certainty for U-MSC in locomotion recovery, medium for BM-MSC in pain alleviation, and low for OPCs in locomotion recovery. With moderate-to-high certainty, our study demonstrated superior efficacy of mesenchymal stem cells, particularly U-MSCs, when administered immediately post-injury at lower doses without antibiotics for locomotion recovery and BM-MSCs for pain alleviation. These findings suggest further clinical investigation of these stem cell types under optimal conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


