家蚕甲基转移fa结构域的进化历史及其在JH信号中的作用
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
甲基转移法尼松酸(MtFA)结构域是法尼松酸o -甲基转移酶(FAMeT)的特征结构域。FAMeT是甲壳类动物的一种关键酶,它催化了法尼索酸甲酯(methyl farnesoate, MF)生物合成的限速步骤。虽然FAMeT的同系物已在昆虫中广泛发现,但它们在幼年激素(JH)信号通路中的作用尚不清楚。此外,MtFA结构域的起源和进化历史仍然知之甚少。有趣的是,昆虫的FAMeT含有功能未知的MtFA结构域和DUF3421结构域。在本研究中,我们发现MtFA结构域广泛分布于不同的分类群中,包括原生动物、脊椎动物和被子植物。含有MtFA结构域的基因可能起源于原核生物和真核生物的共同祖先,但在古细菌中丢失了。MtFA序列的聚类与MtFA融合蛋白的分类基本一致,表明结构域变异可能与蛋白类型有关。BmFAMeT-2(编码含有2个MtFA结构域和1个DUF3421结构域的蛋白)或BmFAMeT-1(编码含有1个MtFA结构域和1个DUF3421结构域的蛋白)的过表达导致bmpr -h1表达上调。相比之下,BGIBMGA006319(编码仅含有1个DUF3421结构域的蛋白)的过表达导致BmKr-h1表达下调。敲低BGIBMGA006319导致JH滴度升高,而敲低BmFAMeT-1或BmFAMeT-2导致JH滴度降低,且BmFAMeT-2的效果比BmFAMeT-1更明显。这些结果表明,MtFA结构域在家蚕JH信号传导中起促进作用,而DUF3421结构域在家蚕JH信号传导中起抑制作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
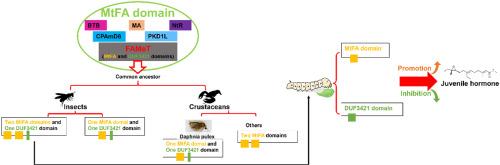
The evolutionary history of the Methyltransf_FA domain and its role in JH signal in silkworm, Bombyx mori
The Methyltransf_farnesoic acid (MtFA) domain is a characteristic domain of farnesoic acid O-methyltransferase (FAMeT). FAMeT serves as a key enzyme in crustaceans, catalyzing the rate-limiting step in the biosynthesis of methyl farnesoate (MF). Although homologs of FAMeT have been widely identified in insects, their role in the juvenile hormone (JH) signaling pathway remains unclear. Moreover, the origin and evolutionary history of the MtFA domain remain poorly understood. Interestingly, insect FAMeT contains the MtFA domain and the DUF3421 domain of unknown function. In this study, we found that the MtFA domain was widely distributed across diverse taxa, including protists, vertebrates, and angiosperms. MtFA domain–containing genes likely originated from the common ancestor of prokaryotes and eukaryotes but were lost in archaea. The clustering of MtFA sequences was generally consistent with the classification of MtFA fusion proteins, indicating that domain variation might have been related to protein type. Overexpression of BmFAMeT-2 (which encodes a protein containing 2 MtFA domains and 1 DUF3421 domain) or BmFAMeT-1 (which encodes a protein containing 1 MtFA domain and 1 DUF3421 domain) led to the upregulation of BmKr-h1 expression. In contrast, overexpression of BGIBMGA006319 (encoding a protein containing only 1 DUF3421 domain) led to the downregulation of BmKr-h1 expression. Knockdown of BGIBMGA006319 led to an increase in JH titers, whereas knockdown of BmFAMeT-1 or BmFAMeT-2 resulted in a decrease, with the effect being more pronounced for BmFAMeT-2 than for BmFAMeT-1. These findings suggest that the MtFA domain plays a promoting role in JH signaling, while the DUF3421 domain plays an inhibitory role in JH signaling in silkworms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


