PROX1在小鼠Corti器官的细胞模式、神经支配和分化中是必需的
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
Corti的器官被分为负责听力或耳蜗放大的功能室。内侧室包含由I型螺旋神经节神经元支配的内毛细胞,外侧室包含由II型螺旋神经节神经元支配的外毛细胞。支持细胞也不同,侧室柱细胞和德特氏细胞发育专门的细胞结构来支持外毛细胞的电运动性。由于Prox1是第一个在侧室支持细胞中严格表达的转录因子,我们培育了皮质限制性Prox1条件敲除小鼠的器官来研究侧室发育。在缺乏Prox1的情况下,外毛细胞的支持细胞数量增加,但没有相应的变化,从形态学标准来看,外毛细胞表现为未完全分化。外毛细胞数量未受影响,但神经支配被破坏,许多传入神经元不正确地转向耳蜗尖。RNAseq显示,基因表达没有变化,可以解释神经支配表型。因此,我们提出PROX1促进柱状细胞和Deiters细胞的分化和组织,这对神经支配有次要影响,但不是隔室规范所必需的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
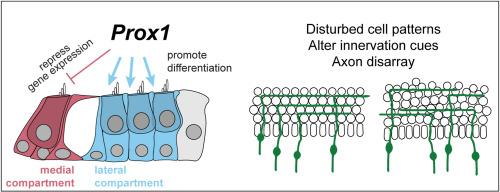
PROX1 is necessary for cellular patterning, innervation and differentiation in the mouse organ of Corti
The organ of Corti is divided into functional compartments responsible for hearing or cochlear amplification. A medial compartment containing inner hair cells innervated by Type I spiral ganglion neurons and a lateral compartment containing outer hair cells innervated by Type II spiral ganglion neurons. Supporting cells also differ, with lateral compartment pillar cells and Deiters' cells developing specialized cellular structures to support outer hair cell electromotility. We bred organ of Corti-restricted Prox1 conditional knockout mice to study lateral compartment development because PROX1 is the first transcription factor expressed strictly in lateral compartment supporting cells. In the absence of Prox1, supporting cell numbers increased without corresponding changes in outer hair cells, and they appear incompletely differentiated based on morphological criteria. Outer hair cell number was not impacted but innervation was disrupted with many afferent neurons turning incorrectly towards the cochlear apex. RNAseq revealed no changes in gene expression that could account for the innervation phenotype. Therefore, we propose that PROX1 promotes pillar and Deiters’ cell differentiation and organization that has secondary effects on innervation but is not required for compartment specification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


