利用RPA-CRISPR/Cas12a技术快速视野检测山药组织中的咖啡叶柄虫
IF 2.5
2区 农林科学
Q1 AGRONOMY
引用次数: 0
摘要
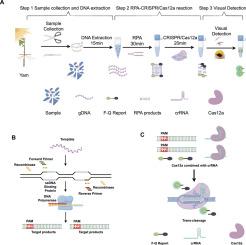
山药(薯蓣属)是世界范围内具有重要经济价值的重要作物。然而,咖啡叶线虫是引起山药根病变的最普遍的致病线虫之一,主要通过种子山药(顶部块茎部分)或土壤传播。确保种子薯蓣的质量和栽培土壤不受致病线虫的侵害是薯蓣生产的关键,而一种有效的检测方法至关重要,特别是在田间条件下。2022年11月至2024年11月,在不需要昂贵的实验室设备和专业人员的情况下,开发了一种简单、快速、高效的山药组织中咖啡假单胞菌分子鉴定方法。整个检测过程在65 min内完成,包括粗线虫DNA提取(10 min)、重组酶聚合酶扩增(RPA) (30 min)和基于CRISPR/ cas12的检测(25 min)。与PCR和qPCR方法相比,该方法灵敏度高,每个反应的检出率低至4拷贝/μL,对咖啡假丝虫具有较高的特异性,与其他常见植物寄生线虫无交叉反应性。采用RPA-CRISPR/Cas12a方法对18种薯蓣种子进行检测,结果与传统PCR检测结果高度一致,表明该方法具有较高的田间检测适用性和准确性。综上所述,RPA-CRISPR/Cas12a作为一种可靠的快速诊断工具具有巨大的潜力,可用于检测咖啡链球菌感染,特别是在没有复杂设备的野外条件下进行山药质量控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rapid visual field detection of Pratylenchus coffeae in yam tissues via RPA-CRISPR/Cas12a
Yam (Dioscorea spp.) is an important crop cultivated worldwide with significant economic value. However, Pratylenchus coffeae is one of the most prevalent pathogenic nematodes causing yam root lesion disease, primarily spreading through seed yams (top tuber portions) or soil. Ensuring that quality of seed yams and cultivated soil free from pathogenic nematodes is crucial for yam production, and an efficient detection method for P. coffeae is essential, especially under field conditions. From November 2022 to November 2024, a simple, rapid, and efficient molecular identification method was developed for P. coffeae detection in yam tissues without requiring expensive laboratory equipment or specialized personnel. The entire detection process was completed in 65 min, including crude nematode DNA extraction (10 min), recombinase polymerase amplification (RPA) (30 min), and CRISPR/Cas12a-based detection (25 min). Compared with PCR and qPCR assays, the proposed diagnostic method demonstrated high sensitivity, with detection as low as 4 copies/μL per reaction, and exhibited high specificity for P. coffeae without cross-reactivity with other common plant-parasitic nematodes. The detection results obtained using RPA-CRISPR/Cas12a were highly consistent with those of conventional PCR assays conducted on 18 seed yams, indicating the method's high applicability and accuracy for field detection. In conclusion, RPA-CRISPR/Cas12a holds great potential as a reliable and rapid diagnostic tool for detecting P. coffeae infections, particularly for seed yam quality control in field conditions where complex equipment is unavailable.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Crop Protection
农林科学-农艺学
CiteScore
6.10
自引率
3.60%
发文量
200
审稿时长
29 days
期刊介绍:
The Editors of Crop Protection especially welcome papers describing an interdisciplinary approach showing how different control strategies can be integrated into practical pest management programs, covering high and low input agricultural systems worldwide. Crop Protection particularly emphasizes the practical aspects of control in the field and for protected crops, and includes work which may lead in the near future to more effective control. The journal does not duplicate the many existing excellent biological science journals, which deal mainly with the more fundamental aspects of plant pathology, applied zoology and weed science. Crop Protection covers all practical aspects of pest, disease and weed control, including the following topics:
-Abiotic damage-
Agronomic control methods-
Assessment of pest and disease damage-
Molecular methods for the detection and assessment of pests and diseases-
Biological control-
Biorational pesticides-
Control of animal pests of world crops-
Control of diseases of crop plants caused by microorganisms-
Control of weeds and integrated management-
Economic considerations-
Effects of plant growth regulators-
Environmental benefits of reduced pesticide use-
Environmental effects of pesticides-
Epidemiology of pests and diseases in relation to control-
GM Crops, and genetic engineering applications-
Importance and control of postharvest crop losses-
Integrated control-
Interrelationships and compatibility among different control strategies-
Invasive species as they relate to implications for crop protection-
Pesticide application methods-
Pest management-
Phytobiomes for pest and disease control-
Resistance management-
Sampling and monitoring schemes for diseases, nematodes, pests and weeds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


