非贵金属Fe3C/Cu电催化脱除硝酸盐:性能、机理及实际水处理性能
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
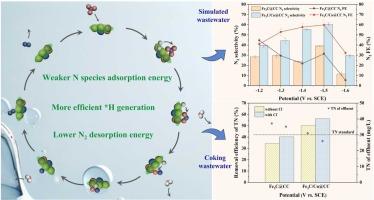
反应过程中总氮(TN)去除率低,阻碍了电催化技术在硝酸盐(NO3-)脱除中的应用。本研究提出了一种非贵金属Fe3C/Cu@CC阴极,通过在碳布上原位生长Fe3C,然后电沉积Cu纳米颗粒,用于电催化还原NO3-。电化学测试表明,Fe3C/Cu@CC具有良好的NO3还原性能和N2选择性,特别是在中性pH条件下。在-1.5 V vs. SCE条件下,模拟废水在5小时内NO3-去除率为68.35%,N2选择性为60.40%。在实际焦化废水处理中,Fe3C/Cu@CC对NO3-的去除率为56.10%,对总氮(TN)的去除率为56.33%,对N2的选择性高达100%。通过UPS、EPR、dem和DFT计算的机理研究表明,Fe-Cu界面处电子密度的重新分布促进了*H的形成,降低了N2在界面处的脱附能垒,进一步提高了N2的选择性。材料表征和循环试验证明,Fe3C/Cu@CC电极在模拟废水和实际焦化废水中都具有良好的稳定性。这项工作为设计有效的非贵金属催化剂用于实际的NO3-废水处理提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrocatalytic Removal of Nitrate by Non-noble Fe3C/Cu: Property, Mechanism, and Practical Water Treatment Performance
The application of electrocatalytic technology in nitrate (NO3-) removal is hindered by the low total nitrogen (TN) removal efficiency during the reaction. In this study, a non-noble Fe3C/Cu@CC cathode, prepared by in-situ growth of Fe3C on carbon cloth followed by electrodeposition of Cu nanoparticles, was proposed for electrocatalytic reduction of NO3-. Electrochemical tests showed that Fe3C/Cu@CC possessed good NO3- reduction property and N2 selectivity, especially under neutral pH condition. 68.35% of NO3- was removed and 60.40% of N2 selectivity was achieved in simulated wastewater at -1.5 V vs. SCE, in 5 hours. For actual coking wastewater treatment, Fe3C/Cu@CC removed 56.10% of NO3- and 56.33% of total nitrogen (TN), with N2 selectivity as high as 100%. Mechanism studies via UPS, EPR, DEMS and DFT calculations revealed the redistribution of electron density at the Fe-Cu interface enhanced the formation of *H and decreased the desorption energy barrier of N2 at the interface, further improving the selectivity of N2. The material characterization and cyclic test proved the good stability of Fe3C/Cu@CC electrode in both simulated wastewater and actual coking wastewater. This work provided insights into efficient non-noble metal catalyst design for practical NO3- wastewater treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


