e -钙粘蛋白在气道中的破坏导致发育不良的应激细胞和哮喘样表型
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
虽然e -钙粘蛋白是粘附体连接的已知核心成分,但它如何控制细胞命运仍然知之甚少。本研究表明,在小鼠气道中,上皮祖细胞俱乐部细胞(而非终末分化纤毛细胞)中e -钙粘蛋白的失活导致增殖增加、杯状细胞化生和免疫浸润,模拟未暴露于过敏原的哮喘表型。单细胞RNA测序鉴定了突变气道的细胞应激特征;先前与纤维化肺中发育不良的肺泡移行细胞相关的特征。趋化因子基因如Cxcl17在体内和分离培养时都在突变气道中上调,这表明上皮具有感知连接破坏和启动免疫防御的内在能力。突变体中Il4ra的失活减弱了杯状细胞化生,但没有免疫募集。总之,这些发现表明,e -钙粘蛋白的缺失通过上皮-内在机制和上皮-免疫串扰导致气道重塑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
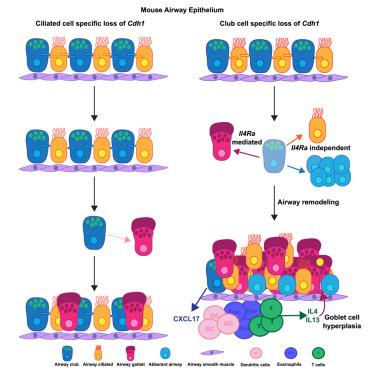
Disruption of E-cadherin in the airway led to dysplastic stressed cells and asthma-like phenotypes
While E-cadherin is a known core component of the adherens junction, how it controls cell fate remains poorly understood. Here, we show that in the mouse airway, inactivation of E-cadherin in epithelial progenitor club cells, but not terminally differentiated ciliated cells, led to increased proliferation, goblet cell metaplasia, and immune infiltration, mimicking asthma phenotypes without exposure to allergen. Single-cell RNA sequencing identified a cellular stress signature in the mutant airway; a profile previously associated with dysplastic alveolar transitional cells in fibrotic lungs. Chemokine genes such as Cxcl17 are upregulated in the mutant airway both in vivo and when cultured in isolation, identifying an intrinsic ability of the epithelium to sense junction breach and launch immune defense. Inactivation of Il4ra in the mutant attenuated goblet cell metaplasia, but not immune recruitment. Together, these findings demonstrate that loss of E-cadherin leads to airway remodeling through both an epithelium-intrinsic mechanism and epithelium-immune crosstalk.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


