末端炔的二碘三氟甲氧基化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
鉴于三氟甲氧基(OCF3)基团的独特性质和烯烃的广泛用途,合成三氟甲氧基化烯烃,特别是含卤素取代基的烯烃具有重要意义。虽然氯/溴三氟甲氧基化烯烃已经建立,但碘变体仍然不发达(单个例子,收率为18%)。本文提出了末端炔的二碘三氟甲氧基化反应,以61-92%的收率生成1,1-二碘-2-(OCF3)-烯烃。该方法具有广泛的底物范围和可扩展性,并能够实现生物活性分子的后期功能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
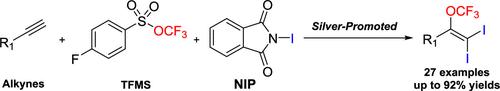
Diiodotrifluoromethoxylation of Terminal Alkynes
Given the unique properties of the trifluoromethoxy (OCF3) group and the broad utility of alkenes, synthesis of trifluoromethoxylated alkenes, particularly those incorporating halogen substituents, is of significant importance. While chloro-/bromo-trifluoromethoxylated alkenes are established, iodo variants remain underdeveloped (single example, 18% yield). We herein present diiodotrifluoromethoxylation of terminal alkynes, delivering 1,1-diiodo-2-(OCF3)-alkenes in 61–92% yields. This method features a broad substrate scope and scalability and enables late-stage functionalization of bioactive molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


