构建全碳四元中心的碘酰化碳二氮化反应。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过多键结构构建全碳四元中心一直是合成化学中的一个重大挑战。在本文中,我们报道了一个串联二芳化过程,通过[2 + 2]环加成在芳族和碘基之间形成四元碘环丁烷中间体。该中间体随后经历1,2-芳基从碘向碳的迁移,产生不对称α-二芳化丙二酸酯。该工艺具有温和的条件,广泛的底物相容性,以及在一步中形成两个C-C键和一个C-I键的能力。这项工作还揭示了碘化试剂的一种新的反应模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
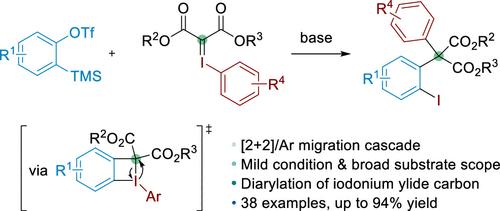
Carbon Diarylation of Iodonium Ylides for Construction of All-Carbon Quaternary Centers
The construction of all-carbon quaternary centers via multiple bond formations remains a significant challenge in synthetic chemistry. Herein, we report a tandem diarylation process initiated via [2 + 2] cycloaddition between aryne species and iodonium ylides to form four-membered iodocyclobutane intermediate. This intermediate subsequently undergoes 1,2-aryl migration from iodonium to carbon, producing unsymmetrical α-diarylated malonic esters. The process is remarkable for its mild conditions, broad substrate compatibility, and capability to form two C–C bonds and one C–I bond in a single step. This work also uncovers a novel reaction mode for iodonium ylide reagents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


