通过胺酮、芳基异硫氰酸酯和单质硫的环化组装噻唑-2-硫酮
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
噻唑-2-硫酮的合成通常具有挑战性,因为它需要使用易燃、有毒的CS2作为硫源。已知的只有几个例子,其中单质硫为噻唑-2-硫酮提供硫原子。然而,这些方法经常受到强氧化剂的过量化学计量量和底物范围的限制。在这里,我们开发了一种方法,允许三组分环胺酮,芳基异硫氰酸酯和元素硫靶向5-苯甲酰噻唑-2-硫酮。我们的成功特点是一种新的方法,在没有外部氧化剂的帮助下,利用胺酮中相对富电子的CC键来提供噻唑-2-硫酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
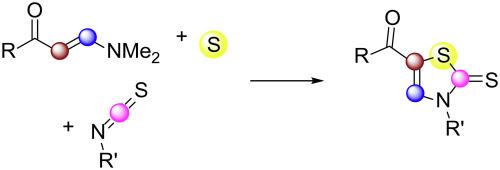
Assembly of thiazole-2-thiones through cyclization of enaminones, aryl isothiocyanates, and elemental sulfur
Synthesis of thiazole-2-thiones is often challenging as it requires the use of flammable, toxic CS2 as the sulfur source. Only a couple of examples in which elemental sulfur provides sulfur atoms for thiazole-2-thiones are known. Yet, those methods often suffer from over-stoichiometric amount of a strong oxidant and the limitation of substrate scope. Herein we develop a method to allow for a three-component annulation of enaminones, aryl isothiocyanates, and elemental sulfur to target 5-benzoylthiazole-2-thiones. Our successes feature a new approach in which relatively electron-rich C![]() C bonds in enaminones are utilized to afford thiazole-2-thiones without the assistance of external oxidants.
C bonds in enaminones are utilized to afford thiazole-2-thiones without the assistance of external oxidants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


