马特森同源条件下叔硼酯的迁移烯化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
叔苄基硼酯在标准mattson同源化条件下使用锂化二氯甲烷进行新的烯化。机理研究表明,相应的α-氯硼酯经过1,2-芳基迁移形成烯烃,形成反应性的碳中间体。这种转化提供了一种罕见的和温和的方法,通过羰基中间体从芳基叔硼酯中获得烯烃。本文章由计算机程序翻译,如有差异,请以英文原文为准。
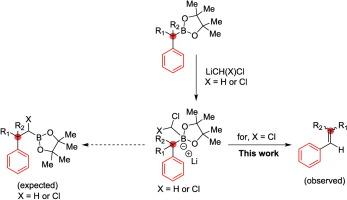
Migratory olefination of tertiary boronic esters under Matteson homologation conditions
Tertiary benzylic boronic esters undergo a novel olefination under standard Matteson homologation conditions using lithiated-dichloromethane. Mechanistic studies reveal the formation of a reactive carbene intermediate from the corresponding α-chloro‑boronic ester that undergoes 1,2-aryl migration to form alkenes. This transformation offers a rare and mild approach to access alkenes from aryl tertiary boronic esters via a carbene intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


