橘皮素通过下调MAOA抑制炎症反应,保护肾脏免受缺血再灌注损伤
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
目的探讨天然抗氧化剂橘皮素对急性肾损伤(AKI)的保护作用及其机制。方法首先建立RIRI小鼠模型,研究橘皮素的肾保护作用。通过RNA测序和网络药理学分析确定潜在的治疗靶点,随后使用GEO和KIT数据库进行验证。接下来,利用分子对接和分子动力学模拟研究了关键靶点和配体之间的相互作用。最后,采用RT-qPCR、免疫组织化学、Western blotting和药理学抑制实验来验证这些靶点的表达谱和功能作用,以及它们在相关信号通路中的参与。结果吉列素能明显改善小鼠的肾功能,减轻RIRI小鼠的组织病理损伤。单胺氧化酶A (MAOA)被认为是一个潜在的治疗靶点,并且在RIRI中被发现显着上调,这被公共数据库分析证实。分子对接和分子动力学模拟表明,橙皮素与MAOA之间存在稳定的结合相互作用。实验验证橘皮素抑制MAOA/NF-κB信号通路,有效减少肾细胞凋亡。此外,橙皮素对肾功能、炎症和MAOA调节的保护作用与MAOA抑制剂氨酰环丙胺相似。结论橘皮素通过抑制MAOA/NF-κB通路,发挥抗炎、抗凋亡作用,减轻riri所致肾损伤。这些发现突出了其作为一种有希望的缺血性AKI治疗候选药物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
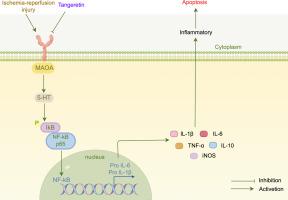
Tangeretin protects the kidney from ischemia-reperfusion injury by inhibiting inflammatory response through downregulation of MAOA
Objective
This study investigates the protective effects and mechanisms of the natural antioxidant tangeretin in alleviating renal ischemia-reperfusion injury (RIRI), a major cause of acute kidney injury (AKI).
Methods
Firstly, a RIRI mouse model was established to investigate the renoprotective effects of tangeretin. Potential therapeutic targets were identified through RNA sequencing and network pharmacology analysis and subsequently validated using the GEO and KIT databases. Next, the interactions between key targets and ligands were examined using molecular docking and molecular dynamics simulations. Finally, RT-qPCR, immunohistochemistry, Western blotting, and pharmacological inhibition assays were used to verify the expression profiles and functional roles of these targets as well as their involvement in relevant signaling pathways.
Results
Tangeretin significantly improved renal function and attenuated histopathological damage in mice subjected to RIRI. Monoamine oxidase A (MAOA) was identified as a potential therapeutic target and was found to be markedly upregulated in RIRI, as confirmed by public database analyses. Molecular docking and molecular dynamics simulations revealed the stable binding interaction between tangeretin and MAOA. Experimental validation demonstrated that tangeretin inhibited MAOA/NF-κB signaling and effectively reduced renal cell apoptosis. Moreover, the protective effects of tangeretin on renal function, inflammation, and MAOA regulation were similar to those of the MAOA inhibitor tranylcypromine.
Conclusion
Tangeretin attenuates RIRI-induced renal injury by inhibiting the MAOA/NF-κB pathway and exerting anti-inflammatory and anti-apoptotic effects. These findings highlight its potential as a promising therapeutic candidate for ischemia-associated AKI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


