内皮细胞来源决定了促炎环境中纤溶标志物的表达和释放
IF 3.4
3区 医学
Q2 HEMATOLOGY
Research and Practice in Thrombosis and Haemostasis
Pub Date : 2025-07-01
DOI:10.1016/j.rpth.2025.102929
引用次数: 0
摘要
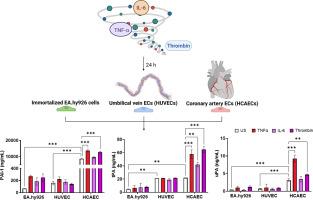
内皮细胞(ECs)为分子相互作用提供了一个表面,分泌各种控制止血的因子。炎症细胞因子可以扰乱血管微环境,可能导致内皮功能障碍和止血失调。目的探讨不同血管床内皮细胞的纤溶平衡及其对促炎刺激的反应。方法分别在静脉(2.5 dyne/cm2)和动脉(12 dyne/cm2)剪切应力下培养人脐静脉内皮细胞(HUVECs)、冠状动脉内皮细胞(HCAECs)和永生化EA.hy926细胞。用凝血酶、白细胞介素6或肿瘤坏死因子-α刺激ECs 24小时。用定量聚合酶链反应测定凝血蛋白和纤溶蛋白的表达,用ELISA或活性测定测定分泌蛋白的表达。在405 nm处监测ECs±300 pM组织型纤溶酶原激活剂(tPA)的血浆凝块溶解情况。结果hcaec的c反应蛋白、尿激酶纤溶酶原激活剂(uPA)和纤溶酶原激活剂抑制剂-1 (PAI-1)的基础分泌量高于HUVECs,但tPA在两者中相似。TNF-α刺激hcaec可增加tPA、uPA和PAI-1的分泌。培养基中tPA/PAI-1和uPA/PAI-1水平较高,游离活性PAI-1水平也较高。相比之下,HUVECs的刺激并没有显著改变基因/蛋白水平。与无细胞对照相比,hcaec和HUVECs使凝块溶解延迟11±8分钟(P <;.01)和8±6分钟(P <;.05),但通过中和PAI-1进行归一化。TNF-α刺激hcaec以pai -1依赖的方式延长凝块溶解。结论hcaec对促炎环境的反应比HUVECs更强,改变了纤溶蛋白的表达,促进了低纤溶反应。这些数据强调hcaec作为冠状血管模型具有筛选新型抗血栓策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Endothelial cell source dictates the expression and release of fibrinolytic markers in a proinflammatory environment
Background
Endothelial cells (ECs) provide a surface for molecular interactions, secreting various factors that govern hemostasis. Inflammatory cytokines can perturb the vascular microenvironment, potentially causing endothelial dysfunction and dysregulation of hemostasis.
Objectives
To examine the fibrinolytic balance of ECs from different vascular beds and their response to proinflammatory stimuli.
Methods
Primary human umbilical vein ECs (HUVECs), human coronary artery ECs (HCAECs), and immortalized EA.hy926 cells were cultured under venous (2.5 dyne/cm2) and arterial (12 dyne/cm2) shear stress. ECs were stimulated with thrombin, interleukin 6, or tumor necrosis factor (TNF)-α for 24 hours. The expression of coagulation and fibrinolytic proteins was quantified by quantitative polymerase chain reaction and secreted proteins via ELISA or activity assay. Plasma clot lysis on ECs ± 300 pM tissue-type plasminogen activator (tPA) was monitored at 405 nm.
Results
Basal secretion of C-reactive protein, urokinase plasminogen activator (uPA), and plasminogen activator inhibitor-1 (PAI-1) was higher in HCAECs than in HUVECs, but tPA was similar in both. TNF-α stimulation of HCAECs increased secretion of tPA, uPA, and PAI-1. Levels of tPA/PAI-1 and uPA/PAI-1 were higher in media, as was free-active PAI-1. In contrast, stimulation of HUVECs did not significantly alter gene/protein levels. HCAECs and HUVECs delayed clot lysis relative to no-cell controls by 11 ± 8 minutes (P < .01) and 8 ± 6 minutes (P < .05) respectively, but were normalized by neutralizing PAI-1. TNF-α stimulation of HCAECs prolonged clot lysis in a PAI-1–dependent manner.
Conclusion
HCAECs respond more potently to a proinflammatory environment than HUVECs, altering expression of fibrinolytic proteins and promoting a hypofibrinolytic response. These data highlight HCAECs as a model of coronary vasculature with potential for screening novel antithrombotic strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Research and Practice in Thrombosis and Haemostasis
Medicine-Hematology
CiteScore
5.60
自引率
13.00%
发文量
212
审稿时长
7 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


