mof包覆FeTiO3:协同抑制锂离子电池阳极材料分解和容量衰退
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
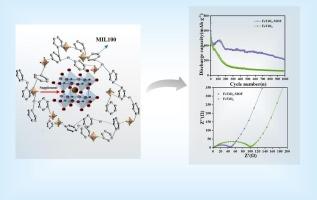
氧化钛铁(FeTiO3)因其独特的八面体结构、天然丰度和较高的理论比容量而成为锂离子电池极具前景的负极材料。然而,在高电流密度下,循环过程中显著的容量衰减和结构不稳定阻碍了实际实施。在本研究中,我们开发了一种创新的溶液浸渍策略,在FeTiO3颗粒(标记为FeTiO3- mof)上构建金属有机框架(MIL-100)保护层。这种工程结构有效地解决了两个关键挑战:(1)通过物理限制抑制活性物质溶解和电极粉碎,(2)通过形成连续导电途径增强电荷转移动力学。优化后的FeTiO3- mof复合材料表现出优异的电化学性能,在0.1 a g−1下循环150次可提供1077.5 mAh g−1的高可逆容量,在1 a g−1下循环1000次可保持222 mAh g−1,是相同条件下原始FeTiO3 (59 mAh g−1)容量的四倍。系统的电化学分析表明,电荷转移特性和锂离子扩散系数显著改善,有效缓解了在高电流密度下通常观察到的快速容量衰减。更重要的是,本研究通过合理利用MOF基体内的金属离子,建立了一种新的自我补充机制,动态补偿长时间循环过程中活性物质的损失。所提出的表面工程策略为开发下一代储能材料提供了一种有效的方法,该材料结合了高容量和卓越的循环稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
MOF-clad FeTiO3: Synergistic suppression of material decomposition and capacity fading in lithium-ion battery anodes
Titanium iron oxide (FeTiO3) has emerged as a promising anode material for lithium-ion batteries due to its distinctive octahedral structure, natural abundance, and high theoretical specific capacity. However, practical implementation has been hindered by significant capacity fading during cycling and structural instability under high current densities. In this study, we developed an innovative solution impregnation strategy to construct a metal-organic framework (MIL-100) protective layer on FeTiO3 particles (denoted as FeTiO3-MOF). This engineered architecture effectively addresses two critical challenges: (1) suppressing active material dissolution and electrode pulverization through physical confinement, and (2) enhancing charge transfer kinetics via the formation of continuous conductive pathways. The optimized FeTiO3-MOF composite demonstrates remarkable electrochemical performance, delivering a high reversible capacity of 1077.5 mAh g−1 after 150 cycles at 0.1 A g−1 and maintaining 222 mAh g−1 after 1000 cycles at 1 A g−1 - quadruple the capacity of pristine FeTiO3 (59 mAh g−1) under identical conditions. Systematic electrochemical analysis reveals significantly improved charge transfer characteristics and lithium-ion diffusion coefficients, effectively mitigating the rapid capacity decay typically observed at elevated current densities. More importantly, this work establishes a novel self-replenishment mechanism through the rational utilization of metal ions within the MOF matrix, which dynamically compensates for active material loss during prolonged cycling. The proposed surface engineering strategy provides an effective method for developing next-generation energy storage materials that combine high capacity with exceptional cycling stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


