成年鲤鱼精子发生和支持细胞可塑性的结构和功能研究
IF 2.5
2区 农林科学
Q3 REPRODUCTIVE BIOLOGY
引用次数: 0
摘要
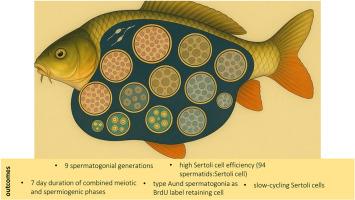
硬骨鱼的精子发生被组织成高度特化的囊性单位,其中支持细胞包裹并支持同步发育的生殖细胞群——这种结构和功能安排与羊膜动物的非囊性精原上皮形成鲜明对比,在羊膜动物的非囊性精原上皮中,支持细胞同时与多个生殖细胞阶段相互作用。在这项研究中,我们对鲤(Cyprinus carpio)的精子发生进行了全面的形态学和体视学分析,鲤是一种具有相当经济意义的水产养殖物种。我们的研究描述了生殖细胞在增殖、减数分裂和生精阶段的发育过程,确定了9个不同的精原细胞世代。支持细胞的支持能力反映在大约94:1的生殖细胞与支持细胞的比例上,强调了这个囊性系统中体细胞投资的效率。使用5 ' -溴-2 ' -脱氧尿苷(BrdU)脉冲追踪方法,我们估计减数分裂和生精过程的总持续时间约为7天。此外,在a型未分化精原细胞和支持细胞群中均检测到长期brdu保留细胞(lrc),表明存在具有延长标签保留的慢循环细胞。空间分析进一步表明,一些精原细胞可能位于小管间室附近,与先前描述的壁龛样区域一致。重要的是,我们发现了一个与生殖细胞囊肿无关的支持细胞样细胞核亚群——这里称为“非囊肿相关”或“自由”支持细胞。虽然它们的确切作用尚不清楚,但它们的形态特征和BrdU标记的保留表明,它们可能参与了生精周期中的结构重塑或囊肿重组。这些观察有助于硬骨鱼睾丸中体细胞可塑性的新观点。总之,我们的发现加强了硬骨鱼囊性精子发生的保守原理,并为生殖细胞与其体细胞环境之间的动态相互作用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural and functional insights into spermatogenesis and Sertoli cell plasticity in adult common carp (Cyprinus carpio)
Spermatogenesis in teleost fishes is organized into highly specialized cystic units, wherein Sertoli cells encapsulate and support cohorts of synchronously developing germ cells—a structural and functional arrangement that contrasts markedly with the non-cystic seminiferous epithelium of amniotes, in which Sertoli cells simultaneously interact with multiple germ cell stages. In this study, we conducted a comprehensive morphological and stereological analysis of spermatogenesis in the common carp (Cyprinus carpio), a species of considerable economic relevance to aquaculture. Our investigation delineated the progression of germ cell development across the proliferative, meiotic, and spermiogenic phases, identifying nine distinct spermatogonial generations. The Sertoli cell support capacity, reflected in a germ-to-Sertoli cell ratio of approximately 94:1, underscores the efficiency of somatic investment in this cystic system. Using a 5′-bromo-2′-deoxyuridine (BrdU) pulse-chase approach, we estimated the combined duration of meiotic and spermiogenic processes to be around seven days. Additionally, long-term BrdU-retaining cells (LRCs) were detected within both type An undifferentiated spermatogonia and Sertoli cell populations, indicating the presence of slow-cycling cells with extended label retention. Spatial analyses further suggested that some spermatogonia may localize near the intertubular compartment, consistent with previously described niche-like regions. Importantly, we identified a subpopulation of Sertoli cell-like nuclei that were not associated with germ cell cysts—herein referred to as “non-cyst-associated” or “free” Sertoli cells. Although their precise role remains to be clarified, their morphological features and retention of BrdU labeling suggest that they may participate in structural remodeling or cyst reassembly during the spermatogenic cycle. These observations contribute to an emerging view of somatic cell plasticity in the teleost testis. Taken together, our findings reinforce the conserved principles of cystic spermatogenesis in teleosts and provide new insights into the dynamic interactions between germ cells and their somatic environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theriogenology
农林科学-生殖生物学
CiteScore
5.50
自引率
14.30%
发文量
387
审稿时长
72 days
期刊介绍:
Theriogenology provides an international forum for researchers, clinicians, and industry professionals in animal reproductive biology. This acclaimed journal publishes articles on a wide range of topics in reproductive and developmental biology, of domestic mammal, avian, and aquatic species as well as wild species which are the object of veterinary care in research or conservation programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


