融合双环n -芳基叠氮嘧啶的制备及区域选择性开环反应。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文系统地评价了双环n -芳基氮杂环的开环反应。这类化合物还没有在开环反应的背景下进行广泛的研究,因此很难预测反应的位置,这可能限制了它们作为合成含氮分子的中间体的使用。我们最近在hunterine A合成中成功的开环策略促使我们使用相关的氮杂环来系统地评估这种转化。我们的研究结果表明,在各种条件下,双环n -芳基叠氮嘧啶的开环反应具有良好的区域选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
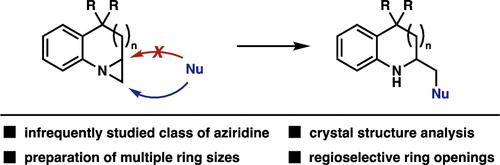
Preparation and Regioselective Ring Opening Reactions of Fused Bicyclic N-Aryl Aziridines
Presented herein is a systematic evaluation of ring opening reactions of bicyclic N-aryl aziridines. This class of compounds has not seen extensive study in the context of ring opening reactions making the site of reaction difficult to predict, potentially limiting their use as intermediates in the synthesis of nitrogen-containing molecules. Our recent successful ring opening strategy in the synthesis of hunterine A prompted us to systematically evaluate this transformation using related aziridines. Our findings show that ring opening reactions of bicyclic N-aryl aziridines occur with exquisite regioselectivity under a variety of conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


