通过镍催化的涉及碳-氧键插入的Suzuki-Miyaura偶联获得苯基甘氨酸衍生的非天然对映体富集氨基酸。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
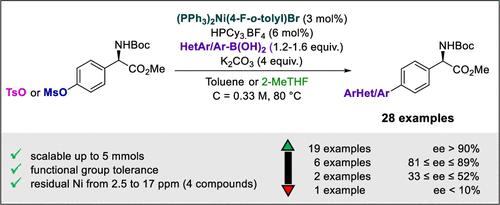
本文报道了镍催化的Suzuki-Miyaura偶联合成非天然双芳基、聚芳基和杂芳基氨基酸衍生物的优化过程(28个例子)。其目的是利用镍对氧基亲电试剂的反应性,提供一种廉价而高效的钯催化反应替代品。为此,使用易于消旋的羟基苯基甘氨酸衍生物。该反应包括空气稳定的镍预催化剂与富电子磷化氢和碳酸钾在无水条件下结合,以确保最高的对映体纯度和转化率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Access to Unnatural Enantioenriched Amino Acids Derived from Phenylglycine through Nickel-Catalyzed Suzuki–Miyaura Coupling Involving Carbon–Oxygen Bond Insertion
We report herein the optimization of a nickel-catalyzed Suzuki–Miyaura coupling for the synthesis of unnatural bi- and polyaryl and heteroaryl amino acid derivatives (28 examples). The aim is to provide an inexpensive and efficient alternative to palladium-catalyzed reactions, exploiting nickel’s reactivity toward oxygen-based electrophiles. To this end, easily racemizable hydroxyphenylglycine derivatives are used. The reaction involves an air-stable nickel precatalyst in association with an electron-rich phosphine and potassium carbonate under water-free conditions, to ensure the highest enantiomeric purities and conversions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


