酯选择性还原为醛:扩展范围和下游反应性。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在我们的研究中,我们使用(2-溴-6-氟苯基)双(2,3,5,6-四氟苯基)硼烷(SBR12)催化剂扩展了酯硅氢化方法的适用性。总的来说,该方法具有极低的催化剂负载(低至0.01 mol %),以及温和的反应条件和对各种官能团的高耐受性。我们通过性信息素(Z,Z)-7,11-十六烯二醛和抗精神病药物氟哌啶醇的简洁全合成证明了该技术的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
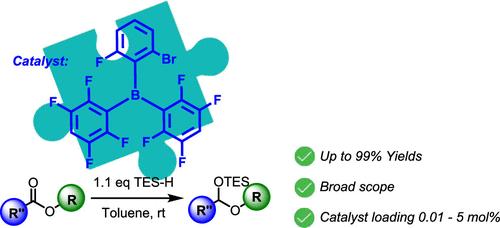
Selective Reductions of Esters to Aldehydes: Extended Scope and Downstream Reactivity
In our study, we extended the applicability of ester hydrosilylation methodology employing (2-bromo-6-fluorophenyl)bis(2,3,5,6-tetrafluorophenyl)borane (SBR12) catalysts. Overall, the method features exceptionally low catalyst loading (as little as 0.01 mol %), along with mild reaction conditions and a high tolerance for various functional groups. We demonstrated the practicality of this technique through the concise total synthesis of the sex pheromone (Z,Z)-7,11-hexadecadienal and the antipsychotic medication haloperidol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


