一种新的靶向LC-MS/MS方法用于心脏淀粉样变性分型的研究
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
背景心脏淀粉样变性(CA)的治疗和预后在很大程度上取决于淀粉样蛋白类型的准确鉴定。组织病理学方法是最常用的方法,但往往产生不确定的结果。基于非靶向蛋白质组学的激光显微解剖质谱法在CA诊断中的应用正逐渐被人们所认识,但其成本高、耗时长,且仍处于科研应用的早期阶段。本研究旨在建立一种基于靶向半定量蛋白质组学的新型分型方法,以解决现有方法的不足。结果对52例CA和52例肥厚性心肌病(HCM)患者进行福尔马林固定、石蜡包埋(FFPE)心肌组织标本分析。建立了以激光显微解剖质谱(LMD-MS)为参比标准的三重四极杆质谱半定量分型方法。共分析了52个多肽。关键淀粉样蛋白(AAPs)——载脂蛋白A-IV (apo A-IV)、载脂蛋白E (apo E)和血清淀粉样蛋白P组分(SAP)的诊断准确率较高,AUC值分别为0.964、0.999和0.923。甲状腺素转运蛋白(TTR)、免疫球蛋白轻链- κ (IGL - κ)和IGL-λ使用标准化评分(NS)进行半量化,调整显微解剖和肽峰面积。NSTTR截断值为0.066区分甲状腺转维蛋白淀粉样变性(ATTR),而NSλ-NSκ区分AL-κ(≤-0.04758)和AL-λ (>0.00999)。该方法成功鉴定出15个ATTR亚型、10个AL-κ亚型、21个AL-λ亚型和混合亚型,与LMD-MS结果一致。相比之下,免疫组织化学准确分型只有14例,大多数结果不确定。意义该靶向半定量质谱法与非靶向LMD-MS分型一致性高,准确率高于免疫组化(100% vs. 30.8%),弥补了非靶向蛋白质组学的不足。它为常规临床实验室的CA分型提供了一种实用的方法,并可能有助于将来识别罕见的淀粉样变亚型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
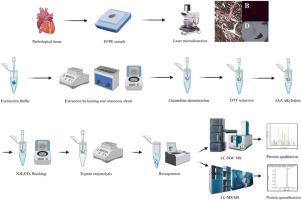
Development of a novel targeted LC-MS/MS methods for the typing of cardiac amyloidosis
Background
The treatment and prognosis of cardiac amyloidosis (CA) depend heavily on the accurate identification of amyloid protein types. Histopathological methods are the most commonly used approach, but often produce inconclusive results. The application of mass spectrometry with laser microdissection mass spectrometry based on non-targeted proteomics in CA diagnosis is gradually being recognized, but it is expensive, time-consuming, and still in the early stages of scientific research applications. This study aims to establish a novel typing method based on targeted semi-quantitative proteomics to address the shortcomings of existing methods.
Results
Formalin-fixed, paraffin-embedded (FFPE) myocardial tissue samples from 52 CA and 52 hypertrophic cardiomyopathy (HCM) patients were analyzed. A semi-quantitative typing method was developed using triple quadrupole mass spectrometry, with laser microdissection mass spectrometry (LMD-MS) serving as the reference standard.
A total of 52 peptides were analyzed. Key amyloid-associated proteins (AAPs) —apolipoprotein A-IV (apo A-IV), apolipoprotein E (apo E), and serum amyloid P component (SAP) — showed high diagnostic accuracy, with AUC values of 0.964, 0.999, and 0.923, respectively. Transthyretin (TTR), immunoglobulin light chains- κ (IGL - κ), and IGL-λ were semi-quantified using normalized scores (NS) adjusted for microdissection and peptide peak areas. An NSTTR cutoff of 0.066 distinguished transthyretin amyloidosis (ATTR), while NSλ-NSκ differentiated AL-κ (≤-0.04758) from AL-λ (>0.00999). The method successfully identified 15 ATTR, 10 AL-κ, 21 AL-λ, and mixed subtypes, aligning with LMD-MS results. By contrast, immunohistochemistry accurately typed only 14 cases, with most results inconclusive.
Significance
This targeted semi-quantitative mass spectrometry method has high consistency with non-targeted LMD-MS typing, with an accuracy higher than IHC (100 % vs. 30.8 %), while compensating for the shortcomings of non-targeted proteomics. It provides a practical method for CA typing in routine clinical laboratories and may help identify rare subtypes of amyloidosis in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


