通过共掺杂Na和N将氮空位和化学吸附位点转化为牛粪生物炭超薄纳米片以去除土环素
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
生物炭因其显著的吸附能力、低廉的成本和丰富的原料,近年来引起了人们对废水处理的极大兴趣。然而,传统生物炭的问题包括相对较小的比表面积和较少的吸附位点,限制了其在实践中的潜在应用。本文采用一步熔盐法合成了不同程度氮缺陷的超薄生物炭纳米片,以去除家畜废水中的典型抗生素土霉素。用氮原子取代碳骨架中的部分碳原子生成含氮官能团(如吡啶- n、吡咯烷- n、石墨- n),显著增强了生物炭的表面极性。同时,钠离子(Na+)与带负电荷的氮位点形成离子对,降低了表面电荷屏蔽效应,增加了生物炭中有效吸附位点的密度。这种协同作用显著提高了生物炭对土霉素的吸附能力,达到82.7 mg/g,是未经处理的生物炭(46.9 mg/g)的1.76倍。此外,其填充柱连续运行速度为311 h,动态吸附量为196.1 mg/g,显示出扩大废水处理工艺的巨大潜力。这种高效吸附过程的主要机制包括氢键、ππ相互作用、静电吸引和孔隙填充。本研究为设计高吸附量、环境友好型吸附剂提供了新思路,并为利用牛粪生物炭处理禽畜废水中的土环素提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

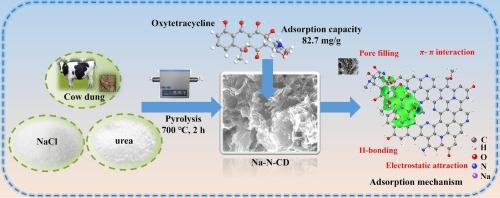
Turning nitrogen vacancy and chemisorption sites by co-doping Na and N into cow dung biochar ultrathin nanosheets for removal of oxytetracycline
Biochar has recently attracted considerable interest in wastewater treatment owing to its remarkable adsorption capabilities, low cost, and abundant raw materials. However, traditional biochar suffers from issues that include a relatively small specific surface area and few adsorption sites, limiting the potential applications in practice. Herein, ultrathin biochar nanosheets with varying degrees of nitrogen defects were synthesized via a one-step molten-salt process to remove oxytetracycline, a typical antibiotic in livestock wastewater. Substituting partial carbon atoms in the carbon skeleton with nitrogen atoms generated nitrogen-containing functional groups (e.g., pyridinic-N, pyrrolic-N, graphitic-N), which significantly enhanced the surface polarity of biochar. Meanwhile, sodium cations (Na+) form ion pairs with negatively charged nitrogen sites, reducing the surface charge shielding effect and increasing the density of effective adsorption sites in biochar. This synergy significantly boosted the biochar’s adsorption capacity for oxytetracycline to 82.7 mg/g, which was 1.76 times that of the untreated biochar at 46.9 mg/g. Furthermore, its packed column consecutively ran for 311 h with a dynamic sorption capacity of 196.1 mg/g, demonstrating significant potential for scaling up wastewater treatment processes. Primary mechanisms underlying this efficient adsorption process encompass H-bonding, π![]() π interaction, electrostatic attraction, and pore-filling. This study provided a novel strategy for designing a high-adsorption capacity, environment-friendly adsorbent and offered a theoretical foundation for treating oxytetracycline in livestock wastewater using biochar derived from cattle dung.
π interaction, electrostatic attraction, and pore-filling. This study provided a novel strategy for designing a high-adsorption capacity, environment-friendly adsorbent and offered a theoretical foundation for treating oxytetracycline in livestock wastewater using biochar derived from cattle dung.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


