Zn2+掺杂水合氧化钒作为阴极:高性能水性锌离子电池的解旋存储机制
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水锌离子电池(azib)由于其低成本、固有的安全性、结构稳定性和低电化学电位而引起了人们的广泛关注。在钒基材料中,V2O5被广泛用作azib的正极材料。然而,结构崩塌和溶解等挑战限制了其循环稳定性。在这项工作中,将商用V2O5剥离,然后通过水热掺杂Zn2+,得到Zn0.34V2O5·2.5H2O (ZnVOH)。当用作阴极时,ZnVOH电极在5a g−1下循环5000次后显示出183.5mAh g−1的比容量。结合一系列的非原位表征,得出Zn2+的储存机理为ZnVOH在放电过程中发生Zn2+/H2O/H+共插层反应,Zn2+和H2O的萃取导致V2O5的非晶化,随后非晶V2O5的部分相转化为Zn3(OH)2V2O7·H2O。不同电解质测试下ZnVOH电极性能的变化表明,水环境可以大大提高ZnVOH电极的容量和稳定性。此外,还以ZnVOH为阴极组装了锌离子混合超级电容器,表现出良好的循环稳定性。该研究为改善Zn2+储存机制和稳定性提供了重要见解,为azib的发展提供了重大进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zn2+ doped hydrated vanadium oxide as cathode: Unravelling storage mechanisms for high-performance aqueous zinc-ion batteries
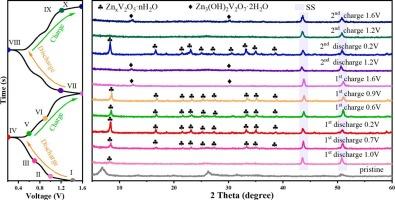
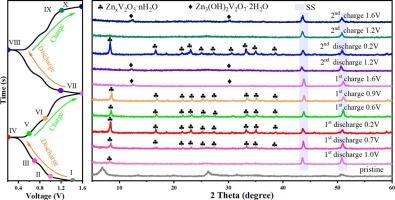
Aqueous zinc-ion batteries (AZIBs) have attracted considerable interest owing to their low cost, inherent safety, structural stability, and low electrochemical potential. Among vanadium-based materials, V2O5 is widely used as a cathode material for AZIBs. However, challenges such as structural collapse and dissolution limit its cycling stability. In this work, commercial V2O5 is exfoliated and subsequently Zn2+ is doped by hydrothermal to obtain Zn0.34V2O5·2.5H2O (ZnVOH). When used as the cathode, the ZnVOH electrode exhibits a specific capacity of 183.5mAh g−1 after 5000 cycles at 5 A g−1. Combined with a series of ex-situ characterizations, the Zn2+ storage mechanism is concluded to be that ZnVOH undergoes Zn2+/H2O/H+ co-intercalation reaction during the discharge process, and the extraction of Zn2+ and H2O leads to the amorphization of V2O5, followed by the partial phase of amorphous V2O5 into Zn3(OH)2V2O7·H2O. The change in performance under different electrolyte tests reveals that the aqueous environment can greatly improve the capacity and stability of the ZnVOH electrode. In addition, a zinc-ion hybrid supercapacitor is assembled with ZnVOH as the cathode, demonstrating excellent cycling stability. This study provides critical insights into improving the mechanism and stability of Zn2+ storage, offering a significant advancement in the development of AZIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


