通过促进早期i型干扰素反应使肿瘤对免疫治疗增敏,使表位扩散
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
癌症免疫治疗的成功是基于靶向高表达的新表位,它优先支持具有高突变负担的恶性肿瘤。在这里,我们表明i型干扰素的早期反应介导免疫检查点抑制剂的成功以及免疫原性较差的肿瘤中的表位扩散,并且这些干扰素反应可以通过系统给药脂质颗粒来增强,脂质颗粒装载了编码肿瘤非特异性抗原的RNA。在小鼠中,对检查点抑制剂敏感的肿瘤的免疫反应可转移到耐药肿瘤,并导致抗原扩散的免疫增强,从而保护动物免受肿瘤的再次攻击。我们的研究结果表明,肿瘤对免疫治疗的抵抗是由缺乏损伤反应决定的,损伤反应可以通过促进早期i型干扰素反应来恢复,从而使治疗难治性肿瘤的表位扩散和自我放大反应成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
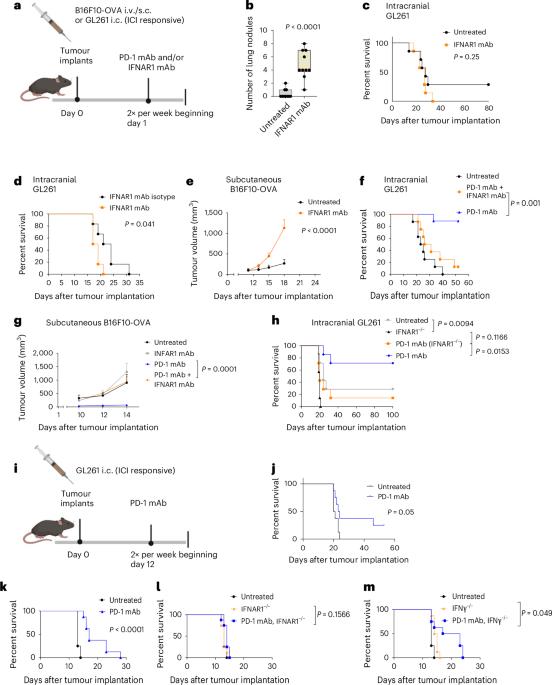
Sensitization of tumours to immunotherapy by boosting early type-I interferon responses enables epitope spreading
The success of cancer immunotherapies is predicated on the targeting of highly expressed neoepitopes, which preferentially favours malignancies with high mutational burden. Here we show that early responses by type-I interferons mediate the success of immune checkpoint inhibitors as well as epitope spreading in poorly immunogenic tumours and that these interferon responses can be enhanced via systemic administration of lipid particles loaded with RNA coding for tumour-unspecific antigens. In mice, the immune responses of tumours sensitive to checkpoint inhibitors were transferable to resistant tumours and resulted in heightened immunity with antigenic spreading that protected the animals from tumour rechallenge. Our findings show that the resistance of tumours to immunotherapy is dictated by the absence of a damage response, which can be restored by boosting early type-I interferon responses to enable epitope spreading and self-amplifying responses in treatment-refractory tumours. Lipid particles loaded with RNA coding for tumour-unspecific antigens can enhance early responses of type-I interferons, mediating the success of immune checkpoint inhibitors as well as epitope spreading.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


