盐形成和聚合物介导的稳定协同方法增强甲苯达唑的生物制药性能。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-14
DOI:10.1016/j.ejpb.2025.114809
引用次数: 0
摘要
甲苯达唑(MBZ)是一种水溶性较差的砖粉分子,在肠道pH值处容易沉淀,阻碍胃肠道吸收。此外,在传统的非晶固体分散体(ASD)中,非晶MBZ在长期储存过程中容易再结晶,降低了其物理稳定性和溶解度。本研究探索了无定形盐固体分散(ASSD),这是一种将盐形成与ASD相结合的新兴方法,其中反离子和聚合物对于形成稳定的过饱和体系至关重要。羟丙基甲基纤维素乙酸琥珀酸酯聚合物的最大过饱和保持能力及其降低MBZ熔融吸热的潜力表明,它适合通过HCl和MBZ之间的酸碱反应制备ASSD。P-XRD和DSC证实了MBZ在ASSD和ASD中的非晶化,ASSD表现出更高的Tg和更好的物理稳定性。1H NMR和FTIR光谱证实了盐形成过程中氮的质子化作用,与ASD相比,更强的盐-聚合物相互作用稳定了ASSD中的无定形MBZ,防止了12 个月的再结晶。此外,asd在MBZ的溶解度(7.58倍)和溶出度(11.17倍)方面表现出协同增强,转化为体内研究,显示Cmax增加2.99倍。因此,研究结果表明,ASD是一种潜在的方法,可以增强MBZ对ASD的生物制药性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
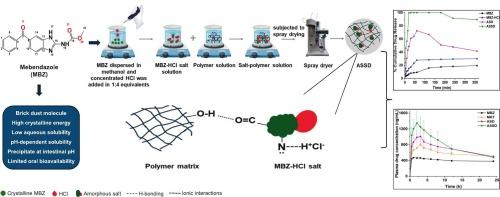
Synergistic approach of salt formation and polymer-mediated stabilization to enhance the biopharmaceutical performance of Mebendazole
Mebendazole (MBZ), a brick dust molecule with poor aqueous solubility tends to precipitate at intestinal pH, impeding its gastrointestinal absorption. Moreover, the amorphous MBZ tends to recrystallize in conventional amorphous solid dispersion (ASD) during long-term storage, reducing its physical stability and solubility. The present investigation explored amorphous salt solid dispersion (ASSD), an emerging approach combining salt formation with ASD, where the counterion and polymer are crucial in forming a stable supersaturated system. The maximal supersaturation holding capacity of hydroxypropyl methylcellulose acetate succinate polymer and its potential to lower the melting endotherm of MBZ demonstrate its suitability for ASSD preparation, via acid-base reaction between HCl and MBZ. P-XRD and DSC confirmed the amorphization of MBZ in ASSD, exhibiting a higher Tg and improved physical stability. 1H NMR and FTIR spectroscopy confirmed the protonation of nitrogen during salt formation, with stronger salt-polymer interactions that stabilized the amorphous MBZ in ASSD, preventing recrystallization for 12 months, compared to ASD. Additionally, ASSD exhibited synergistic enhancement in solubility (7.58-folds) and dissolution (11.17-folds) of MBZ, which translated into in vivo studies, demonstrating 2.99-folds increase in Cmax. Hence, findings revealed ASSD as a potential approach for augmenting the biopharmaceutical performance of MBZ over ASD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


