铜催化非对映选择性α-(n -芳基)氨基烷基化乙烯基芳烃与氮酮。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
已经开发出一种铜催化的乙烯基芳烃、双(pinacolato)二硼和硝基的三组分偶联剂,该偶联剂通过还原处理提供了多种γ-(n -芳基)氨基硼酸盐,具有良好到优异的非对映选择性。这种新方案可以在克尺度上进行,利用氮酮作为简单的前体,并提供在下游应用中具有多种用途的γ-氨基硼酸盐。此外,氧化途径控制允许选择性生成3-取代α,β-不饱和亚胺,扩大了该催化体系的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
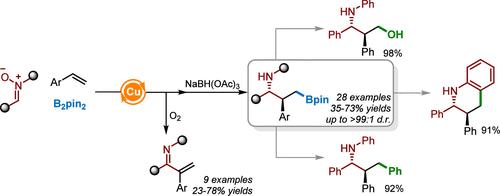
Copper-Catalyzed Diastereoselective Borylative α-(N-Aryl)aminoalkylation of Vinyl Arenes with Nitrones
A copper-catalyzed three-component coupling of vinyl arenes, bis(pinacolato)diboron, and nitrones has been developed, which offers access to a diverse range of γ-(N-aryl)amino boronates via reductive workup, exhibiting good-to-excellent diastereoselectivities. This new protocol, which can be performed at a gram scale, utilizes nitrones as simple precursors and provides γ-amino boronates having versatile utilities in downstream applications. Additionally, oxidative pathway control allows the selective generation of 3-substituted α,β-unsaturated imines, expanding the synthetic utility of this catalytic system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


