TAF1是小鼠胎儿造血所必需的,而不是成年造血所必需的
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
虽然许多序列特异性转录因子(TFs)已被确定为造血干细胞(HSC)谱系确定的关键调节因子,但一般TFs在HSC行为中的功能尚不清楚。为了评估TFIID亚基TAF1在正常造血中的功能,我们制造了TAF1条件敲除(cKO)小鼠,并确定了TAF1在胎儿造血中的重要作用。令人惊讶的是,TAF1缺失对成年小鼠的造血功能没有致命影响;相反,我们观察到造血干细胞和祖细胞(HSPC)区明显扩大,这些细胞的自我更新能力增强,分化能力受损。在嵌合小鼠中,taf1缺失的HSPCs不能产生成熟血细胞;这些细胞在体外诱导分化时也未能上调关键分化基因。TAF1缺失不仅破坏了TFIID染色质募集,还减少了RNA聚合酶II (RNAPII)启动子近端暂停。因此,HSPCs利用不同的转录调控机制进行分化,而不是维持自我更新。本文章由计算机程序翻译,如有差异,请以英文原文为准。
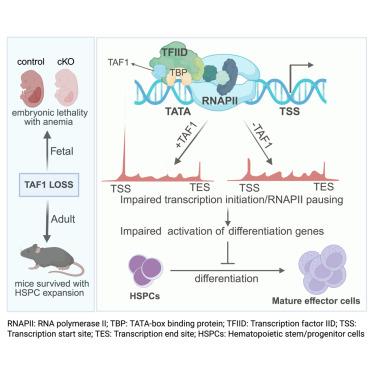
TAF1 is required for fetal but not adult hematopoiesis in mice
While many sequence-specific transcription factors (TFs) have been identified as key regulators of hematopoietic stem cell (HSC) lineage determination, the function of general TFs in HSC behavior is poorly understood. To evaluate the function of the TFIID subunit TAF1 in normal hematopoiesis, we generated Taf1 conditional knockout (cKO) mice and identified an essential role of TAF1 in fetal hematopoiesis. Surprisingly, TAF1 deletion in adult mice was not lethal to hematopoiesis; rather, we observed a marked expansion of the hematopoietic stem and progenitor cell (HSPC) compartment, with increased self-renewal and impaired differentiation capacity of these cells. TAF1-null HSPCs failed to produce mature blood cells in chimeric mice; these cells also failed to upregulate key differentiation genes when induced to differentiate in vitro. TAF1 loss not only disrupted TFIID chromatin recruitment but also reduced RNA polymerase II (RNAPII) promoter-proximal pausing. Thus, HSPCs utilize distinct transcriptional regulatory mechanisms to undergo differentiation versus maintaining self-renewal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


