5-HT2A受体塑造雄性小鼠全脑单胺能一致性。
IF 3.9
2区 医学
Q1 CLINICAL NEUROLOGY
Progress in Neuro-Psychopharmacology & Biological Psychiatry
Pub Date : 2025-08-30
DOI:10.1016/j.pnpbp.2025.111437
引用次数: 0
摘要
由于血清素能致幻剂在各种精神疾病中的临床疗效,其作用机制在世界范围内得到了越来越多的探索。除了刺激5-羟色胺2 A (5-HT2A)受体外,致幻剂可能会使大脑区域之间的活动不同步,然而,相应的神经传递系统的参与在很大程度上被忽视了。考虑到单胺系统几乎针对所有大脑区域并在探索行为中发挥作用,我们假设在小鼠的强迫探索行为中,致幻剂破坏了单胺系统在大脑区域的一致性。通过对死后脑组织中28个不同脑区血清素(5-HT)、多巴胺(DA)、去甲肾上腺素(NA)及其代谢物的定量分析,我们观察到在给药小鼠体内单胺之间存在紧密且高度有组织的相关性。这种组织被致幻剂5-HT2A受体激动剂TCB-2(0.3, 3和10 mg/kg)和拮抗剂MDL-100,907(0.2 mg/kg)破坏,两者都降低了区域神经化学浓度之间的相关性。有趣的是,MDL-100,907和TCB-2的组合部分恢复了相关性。在定量上,TCB-2剂量依赖性地降低了所有脑区5-羟色胺的代谢(代谢物/5-羟色胺),以及纹状体中DA的代谢(3-甲氧基酪胺/DA)。TCB-2还增强了某些大脑区域的DA和NA系统标记物,特别是包括前扣带皮层。MDL-100,907在单独给药时对单胺水平的影响最小,减少了TCB-2(3 mg/kg)诱导的头抽搐和前扣带皮层单胺浓度的增加,但不影响TCB-2诱导的大脑5-羟色胺转换的减少。这些数据表明,在探索过程中,单胺能系统的功能连通性对通过激活或阻断5-HT2A受体进行的调节高度敏感。本文章由计算机程序翻译,如有差异,请以英文原文为准。
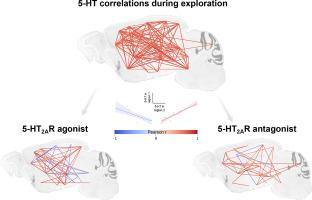
5-HT2A receptors shape whole-brain monoaminergic coherence in male mice
The mechanism of action of serotonergic psychedelics is increasingly explored worldwide due to their clinical benefits in various psychiatric conditions. Beyond the stimulation of serotonin 2A (5-HT2A) receptors, psychedelics may desynchronize activity between brain regions, however the involvement of the corresponding neurotransmission systems has been largely overlooked. Given that monoaminergic systems target virtually all brain regions and play a role in exploratory behavior, we hypothesized that psychedelics disrupt the coherence of monoamine systems across brain regions during forced exploratory behavior in mice. Using post-mortem tissue quantification of serotonin (5-HT), dopamine (DA), noradrenaline (NA), and their metabolites in 28 distinct brain regions, we observed a dense and highly organized pattern of correlations within and between monoamines in vehicle-treated mice. This organization was disrupted by both the psychedelic 5-HT2A receptor agonist TCB-2 (0.3, 3 and 10 mg/kg) and the antagonist MDL-100,907 (0.2 mg/kg), both of which decreased correlations between regional neurochemical concentrations. Interestingly, the combination of MDL-100,907 and TCB-2 partially restored correlations. Quantitatively, TCB-2 dose-dependently decreased 5-HT turnover (metabolite/5-HT) across all brain regions, and DA turnover (3-methoxytyramine/DA) in the striatum. TCB-2 also enhanced markers of the DA and NA systems in certain brain regions, notably including the anterior cingulate cortex. MDL-100,907, which had minimal impact on monoamine levels when administered alone, reduced TCB-2 (3 mg/kg)-induced head twitches and increased monoamine concentrations in the anterior cingulate cortex, but did not affect the TCB-2-induced decrease 5-HT turnover across the brain. These data suggest that the functional connectivity of monoaminergic systems during exploration is highly sensitive to modulation through either activation or blockade of 5-HT2A receptors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.00
自引率
1.80%
发文量
153
审稿时长
56 days
期刊介绍:
Progress in Neuro-Psychopharmacology & Biological Psychiatry is an international and multidisciplinary journal which aims to ensure the rapid publication of authoritative reviews and research papers dealing with experimental and clinical aspects of neuro-psychopharmacology and biological psychiatry. Issues of the journal are regularly devoted wholly in or in part to a topical subject.
Progress in Neuro-Psychopharmacology & Biological Psychiatry does not publish work on the actions of biological extracts unless the pharmacological active molecular substrate and/or specific receptor binding properties of the extract compounds are elucidated.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


