丙基羧酸的电化学硒/碲环化。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了一种新的、可持续的丙炔羧酸硒/碲环化电化学方法,在环境条件下提供了多种有价值的硒/碲结合的不饱和γ-内酯。初步的机制研究表明,这种转化主要通过阳离子途径进行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
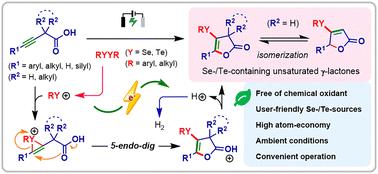
Electrochemical seleno-/tellurocyclization of propargyl carboxylic acids†
Reported herein is a novel and sustainable electrochemical seleno-/tellurocyclization of propargyl carboxylic acids, affording a diverse range of valuable Se-/Te-incorporated unsaturated γ-lactones under ambient conditions. Preliminary mechanistic studies suggest that this transformation predominantly proceeds via a cationic pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


