人类中央凹内视觉感知的同步
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
人类大脑通过处理具有不同时间特征的感觉信号来构建世界模型,这些信号在单一感觉模态中产生和传输速度可能不同。为了感知同时发生的事件,大脑必须同步这些感觉信息,但这种同步的机制尚不清楚。通过结合人类神经记录、行为测量和建模,我们发现,在人类视觉系统中,这一过程始于中央凹,即用于阅读和识别人脸的视网膜区域。对中央凹单锥光刺激的反应时间在整个中央视野范围内是相似的,尽管来自相邻中央凹锥的视觉信息沿不同长度的轴突传播。通过对动作电位传播速度、轴突直径和长度的直接测量,我们发现,越长的中央凹轴突直径越大,传播速度越快。我们的结论是,人类大脑协调视网膜中无髓鞘轴突的轴突传导速度,以同步感觉信号的到达时间。这些结果表明了一种以前未知的人类大脑同步感知的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
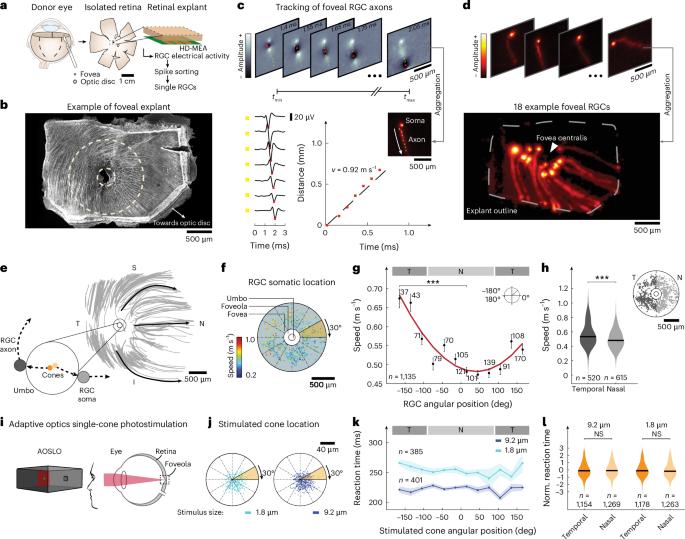

Synchronization of visual perception within the human fovea
The human brain constructs a model of the world by processing sensory signals with distinct temporal characteristics that may differ in generation and transmission speed within a single sensory modality. To perceive simultaneous events as occurring at the same time, the brain must synchronize this sensory information, yet the mechanisms underlying such synchronization remain unclear. By combining human neural recordings, behavioral measurements and modeling, we show that in the human visual system, this process begins in the fovea centralis, the retinal region used for reading and recognizing faces. Reaction times to foveal single-cone photostimulation were similar across the central visual field, although visual information from neighboring foveal cones travels along axons of highly different lengths. From direct measurements of action potential propagation speeds, axon diameters and lengths in the human fovea centralis, we found that longer foveal axons have larger diameters and increased propagation speeds. We conclude that the human brain orchestrates axonal conduction speeds of unmyelinated axons in the retina to synchronize the arrival times of sensory signals. These results suggest a previously unknown mechanism by which the human brain synchronizes perception. Combining behavioral data, electrophysiology and modeling, the authors show that the human brain synchronizes visual signals by adjusting axonal conduction speed in the retina, revealing a previously unknown mechanism for precise perceptual timing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


