比较单细胞分析揭示了蝙蝠翅膀发育中保守基因程序的进化再利用
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
摘要
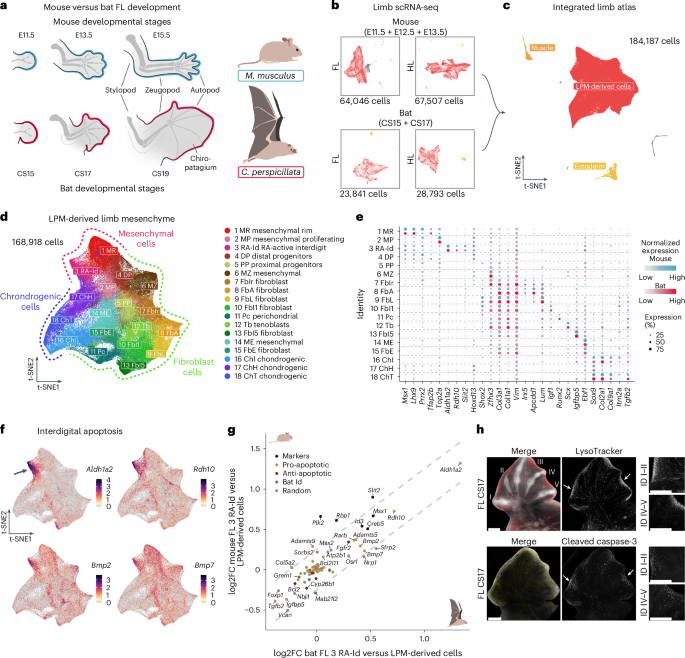
蝙蝠是唯一能够自主飞行的哺乳动物,这是一种基于前肢转变为翅膀的进化创新。蝙蝠翅膀的特点是第二到第五趾的极度伸长,一种叫做指翼的翅膀膜将它们连接起来。在这里,我们通过使用组学工具和单细胞分析比较蝙蝠和小鼠四肢,研究了这种结构的发育和细胞起源。尽管物种之间存在巨大的形态差异,但我们观察到细胞群体和基因表达模式的总体守恒,包括指间凋亡。单细胞分析鉴定了一个特定的成纤维细胞群体,独立于凋亡相关的指间细胞,作为该组织的起源。这些远端细胞表达保守的基因程序,包括转录因子MEIS2和TBX3,这些转录因子通常被认为指定和模式早期近端肢体。MEIS2和TBX3在小鼠远端肢体细胞中的转基因异位表达,激活了翅膀发育过程中表达的基因,并导致翅膀形态相关的表型变化,如趾融合。我们的研究结果阐明了蝙蝠翅膀发育的基本分子机制,并说明了在进化过程中如何通过重新利用现有的发育程序来实现剧烈的形态变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Comparative single-cell analyses reveal evolutionary repurposing of a conserved gene programme in bat wing development
Bats are the only mammals capable of self-powered flight, an evolutionary innovation based on the transformation of forelimbs into wings. The bat wing is characterized by an extreme elongation of the second to fifth digits with a wing membrane called the chiropatagium connecting them. Here we investigated the developmental and cellular origin of this structure by comparing bat and mouse limbs using omics tools and single-cell analyses. Despite the substantial morphological differences between the species, we observed an overall conservation of cell populations and gene expression patterns including interdigital apoptosis. Single-cell analyses of micro-dissected embryonic chiropatagium identified a specific fibroblast population, independent of apoptosis-associated interdigital cells, as the origin of this tissue. These distal cells express a conserved gene programme including the transcription factors MEIS2 and TBX3, which are commonly known to specify and pattern the early proximal limb. Transgenic ectopic expression of MEIS2 and TBX3 in mouse distal limb cells resulted in the activation of genes expressed during wing development and phenotypic changes related to wing morphology, such as the fusion of digits. Our results elucidate fundamental molecular mechanisms of bat wing development and illustrate how drastic morphological changes can be achieved through repurposing of existing developmental programmes during evolution. Single-cell comparison of developing bat and mouse limbs reveals conservation of cell populations and gene expression patterns, and suggests repurposing of genes involved in proximal limb development for wing evolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


