线粒体呼吸是CD8+ T细胞增殖和细胞命运的必要条件
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
线粒体电子传递链(ETC)功能与ATP的产生有关,ATP是包括活性氧(ROS)、嘧啶和三羧酸循环代谢物在内的信号分子。线粒体电子传递是T细胞增殖所必需的2,3,4。然而,对于CD8+ T细胞反应的每种动态状态,哪些线粒体ETC功能是必需的尚不清楚。在这里,我们报道了线粒体复合体III功能的损害,减少呼吸,与ATP产生和超氧化物产生相关的质子泵,减少外周幼稚数,抗原诱导的CD8+ T细胞增殖和记忆形成。急性刺激线粒体复合物iii缺陷的CD8+ T细胞诱导耗尽样表型。在线粒体复合物iii缺陷的CD8+ T细胞中,表达小肠替代氧化酶(AOX)可以恢复呼吸,而不产生ROS或质子泵,并挽救增殖和耗尽表型,但不能形成初始或记忆。因此,T细胞的发育、增殖和记忆形成对线粒体复合体III ROS有不同的要求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
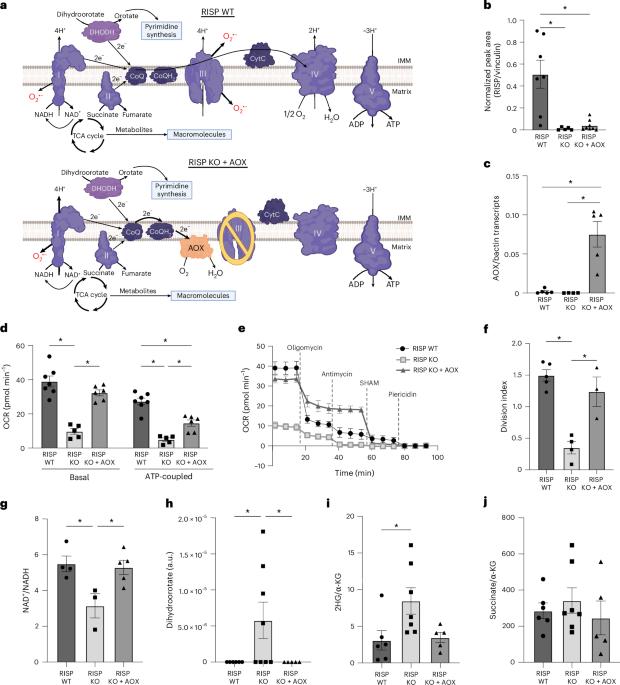

Mitochondrial respiration is necessary for CD8+ T cell proliferation and cell fate
Mitochondrial electron transport chain (ETC) function is linked to the generation of ATP, signaling molecules including reactive oxygen species (ROS), pyrimidines and tricarboxylic acid cycle metabolites1. Mitochondrial electron transport is required for T cell proliferation2–4. However, which mitochondrial ETC functions are necessary for each dynamic state of CD8+ T cell responses is unknown. Here we report that impairing mitochondrial complex III function, which diminishes respiration, proton pumping linked to ATP production and superoxide production, decreases peripheral naive numbers, antigen-induced CD8+ T cell proliferation and memory formation. Acute stimulation of mitochondrial complex III-deficient CD8+ T cells induced an exhausted-like phenotype. Expression of Ciona intestinalis alternative oxidase (AOX) in mitochondrial complex III-deficient CD8+ T cells restores respiration without generating ROS or proton pumping, and rescues proliferation and the exhausted phenotype but not naive or memory formation. Thus, T cell development, proliferation and memory formation have distinct requirements for mitochondrial complex III ROS. Mitochondrial electron transport chain activity provides ATP, generates superoxide, regulates apoptosis and provides the biosynthetic building blocks for growing cells. Here Steinert et al. genetically dissect these functions and find that, in the absence of mitochondrial complex III function, acute stimulation results in CD8+ T cell exhaustion and that mitochondrial complex III reactive oxygen species are required for establishment of naive and memory populations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


