限时喂养调节高脂肪饮食雄性Wistar大鼠脊髓的神经元-胶质相互作用和昼夜节律。
IF 2.2
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
研究设计:本研究利用雄性Wistar大鼠研究限时喂养(TRF)对高脂肪饮食(HFD)诱导的脊髓(T5-T9)神经元-胶质相互作用和基因表达水平改变的影响。目的:评估TRF是否能减轻hfd诱导的脊髓小胶质细胞形态、星形胶质细胞数量、神经周围网络(PNN)完整性、嘌呤能受体表达、炎症和昼夜节律相关基因表达的改变。设置:阿姆斯特丹大学医疗中心,位置AMC,荷兰。方法:雄性Wistar大鼠随机饲喂标准饲料或高脂饲料4周。在此之后,HFD组的大鼠进一步分为两个亚组:继续HFD自由或HFD加TRF再加4周。鼠粮组在实验期间继续自由采食。干预结束时,收集脊髓(T5-T9)进行分析。评估脊髓的小胶质细胞形态、星形胶质细胞数量和PNN完整性。分析嘌呤能受体、炎症和时钟基因的表达水平,以研究神经元-胶质相互作用和昼夜节律稳定。结果:TRF降低了小胶质细胞的激活,保持了PNN的完整性,抑制了hfd诱导的嘌呤能受体上调,稳定了生物钟基因的表达。结论:这些研究结果表明,TRF是一种很有前途的非药物策略,可以对抗致肥性饮食诱导的神经网络降解和神经炎症,突出了其作为基于生活方式的疼痛管理干预的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
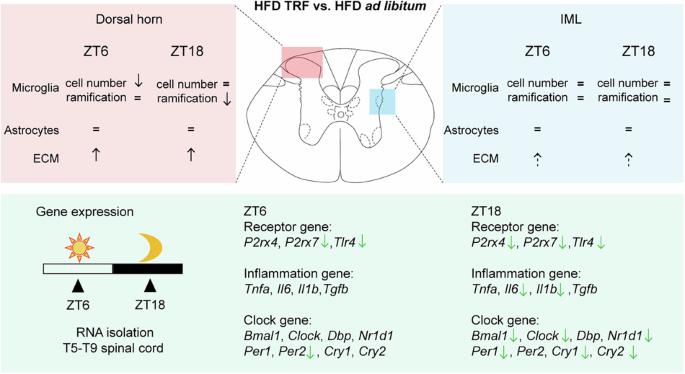
Time-restricted feeding modulates neuron-glial interactions and circadian rhythm in the spinal cord of male Wistar rats fed a high-fat diet
This study utilized male Wistar rats to investigate the effects of time-restricted feeding (TRF) on high-fat diet (HFD)-induced alterations in neuron-glial interactions and gene expression levels in the spinal cord (T5-T9). To evaluate whether TRF mitigates HFD-induced alterations in microglial morphology, astrocyte numbers, perineuronal net (PNN) integrity, purinergic receptor expression, inflammation and circadian rhythm-related gene expression in the spinal cord. Amsterdam University Medical Centers, location AMC, The Netherlands. Male Wistar rats were initially fed either a standard chow diet or a HFD ad libitum for 4 weeks. After this period, rats in the HFD group were further divided into two subgroups: continued HFD ad libitum or HFD with TRF for an additional 4 weeks. Rats in the chow group continued with ad libitum feeding throughout the experimental period. At the end of the intervention, spinal cords (T5–T9) were collected for analysis. Microglial morphology, astrocyte cell numbers, and PNN integrity were assessed in the spinal cord. Expression levels of purinergic receptors, inflammation and clock genes were analyzed to investigate neuron-glial interactions and circadian rhythm stabilization. TRF reduced microglial activation, preserved PNN integrity, suppressed HFD-induced upregulation of purinergic receptors, and stabilized circadian clock gene expression. These findings suggest that TRF is a promising non-pharmacological strategy to counteract obesogenic diet-induced perineuronal net degradation and neuroinflammation, highlighting its potential as a lifestyle-based intervention for pain management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


