组蛋白H3K27去甲基化酶在发育过程中的时空动态。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
时空基因表达是细胞身份和功能的基础,确保正常发育和组织稳态。组蛋白修饰,如H3K4甲基化(与主动转录相关)和H3K27甲基化(与抑制相关),作为微调基因表达的分子开关。然而,在正常发育过程中是否以及如何调节组蛋白修饰酶仍不清楚。在果蝇中,唯一的H3K27去甲基化酶Utx在去除整个基因组中H3K27的二甲基化和三甲基化标记中起着至关重要的作用。在这里,我们提供了第一个证据,证明Utx转录是动态调控的,其调控元件在整个发育过程中表现出不同的时间和空间活动。尽管在转录水平上存在这种可变性,但Utx蛋白普遍表达且相对稳定。我们发现Utx的调控元件在胚胎发生期间高度活跃,但在幼虫和蛹阶段的翅膀、眼睛和腿祖组织中大部分失活。有趣的是,这些调节因素在成年后仍在大脑中持续活跃。破坏这种动态调节激活了一种监视机制,该机制限制了过量的Utx转运到细胞核中,从而确保了最佳的核蛋白水平。此外,虽然Utx的Jumonji C (JmjC)去甲基酶活性对果蝇的生存至关重要,但我们也发现该结构域的完整性对Utx蛋白的表达至关重要。我们的研究结果揭示了Utx调控的一个以前未被认识到的方面,强调了其表达和定位的精确控制如何保护发育过程并维持表观遗传稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
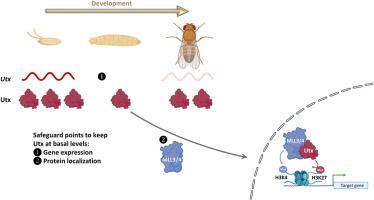
Spatiotemporal dynamics of histone H3K27 demethylase during development
Spatiotemporal gene expression is fundamental to cellular identity and function, ensuring proper development and tissue homeostasis. Histone modifications, such as H3K4 methylation (associated with active transcription) and H3K27 methylation (linked to repression), act as molecular switches that fine-tune gene expression. However, it remains largely unclear whether and how the histone modifying enzymes are regulated during normal development. In Drosophila, Utx, the sole H3K27 demethylase, plays a crucial role in removing di- and trimethylation marks on H3K27 across the genome. Here, we provide the first evidence that Utx transcription is dynamically regulated, with its regulatory elements exhibiting distinct temporal and spatial activity throughout development. Despite this variability at the transcriptional level, Utx protein is ubiquitously expressed and relatively stable. We found that the regulatory elements of Utx are highly active during embryogenesis but become largely inactivated in wing, eye and leg progenitor tissues during larval and pupal stages. Intriguingly, these regulatory elements are persistently active in the brain into adulthood. Disrupting this dynamic regulation activates a surveillance mechanism that limits excess Utx from translocating into the nucleus, thereby ensuring optimal nuclear protein levels. Moreover, while the Jumonji C (JmjC) demethylase activity of Utx is essential for Drosophila viability, we also discovered that the integrity of this domain is crucial for Utx protein expression. Our findings uncover a previously unrecognized aspect of Utx regulation, highlighting how precise control of its expression and localization safeguards developmental processes and maintains epigenetic stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


