利用尖晶石-金属协同作用的MnFe2O4-Co核壳催化剂的可持续海水氧化
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
设计在富含氯化物的海水环境中同时提供高活性和长期稳定性的电催化剂,仍然是开发实用水分解技术的一个巨大挑战。在此,我们报告了一种新型core@shell MnFe2O4-Co CS纳米结构,该结构经过战略设计,可以提高阳极材料在海水电解条件下的析氧反应(OER)性能和耐腐蚀性。core@shell结构有利于MnFe2O4-Co界面上的强电子相互作用,正如XPS和拉曼位移所证明的那样,它们调节了表面和电子结构,增强了电荷转移,降低了OER中间体的能垒。电化学评价表明,MnFe2O4 - co CS具有非常低的起始电位(相对于RHE约1.48 V),较低的Tafel斜率和显著更高的交换电流密度,强调其相对于原始MnFe2O4和co基准具有优越的内在OER动力学。值得注意的是,Co壳在抑制金属离子浸出和结构降解方面起着至关重要的作用,作为一种有效的屏障,防止氯化物引起的腐蚀,确保在海水中长时间运行时的优异稳定性。这些发现阐明了核壳界面工程在调节反应动力学和耐腐蚀性方面的关键作用,为海水氧化提供了化学稳定和动力学有利的表面环境。本文章由计算机程序翻译,如有差异,请以英文原文为准。
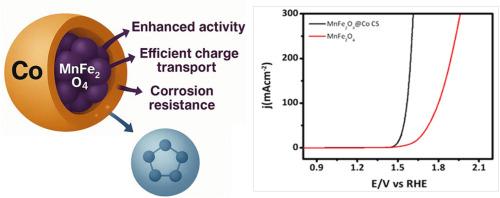
Harnessing spinel–metal synergy in MnFe2O4–Co core shell catalysts for sustainable seawater oxidation
Designing electrocatalysts that simultaneously offer high activity and long-term stability in chloride-rich seawater environments remains a formidable challenge in developing practical water-splitting technologies. Herein, we report a novel core@shell MnFe2O4–Co CS nanostructure strategically engineered to enhance the oxygen evolution reaction (OER) performance and corrosion resistance of anode materials under seawater electrolysis conditions. The core@shell architecture facilitates strong electronic interactions at the MnFe2O4–Co interface, as evidenced by XPS and Raman shifts, which modulate the surface and electronic structure, enhance charge transfer, and lower the energy barrier for OER intermediates. Electrochemical evaluations reveal that MnFe2O4–Co CS exhibits a remarkably low onset potential (∼1.48 V vs. RHE), lower Tafel slope, and significantly higher exchange current density, underscoring its superior intrinsic OER kinetics relative to pristine MnFe2O4 and Co-based benchmarks. Notably, the Co shell plays a critical role in suppressing metal ion leaching and structural degradation by serving as an effective barrier against chloride-induced corrosion, ensuring exceptional stability over prolonged operation in seawater. These findings elucidate the critical role of core–shell interfacial engineering in modulating reaction kinetics and corrosion resistance, offering a chemically stable and kinetically favorable surface environment for seawater oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


