无催化剂和溶剂的多组分合成二氢吡啶从生物质衍生的积木
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
使用生物质衍生的构建块作为石化底物的可持续替代品在精细化学合成中越来越重要。在这项工作中,我们探索了无溶剂和无催化剂的多组分Hantzsch反应,利用生物质醛-香兰素和糠醛合成1,4-和1,2-二氢吡啶。香兰素使产物的选择性生成取决于温度:在动力学控制下,1,2-二氢吡啶在20℃下得到,而在热力学控制下,1,4-二氢吡啶在80℃下占优势。得到的1,2-二氢吡啶对进一步芳构化具有抗性。相反,由于其较高的反应活性,糠醛在任何温度下都能产生1,4-二氢吡啶。共合成了12个化合物,其中3个是新化合物。产量从69%到92%不等。该过程符合绿色化学原则,绿色计量指标如原子经济性(AE)、e因子(EF)和产品质量强度(PMI)证实了这一点。可持续性通过代表性反应的径向五边形图进一步可视化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
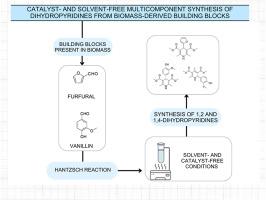
Catalyst- and solvent-free multicomponent synthesis of dihydropyridines from biomass-derived building blocks
The use of biomass-derived building blocks as sustainable alternatives to petrochemical substrates is gaining relevance in fine chemical synthesis. In this work, we explore the solvent- and catalyst-free multicomponent Hantzsch reaction using biomass-based aldehydes—vanillin and furfural—to synthesize 1,4- and 1,2-dihydropyridines.
Vanillin enabled selective product formation depending on temperature: 1,2-dihydropyridines were obtained at 20 °C under kinetic control, while 1,4-dihydropyridines predominated at 80 °C under thermodynamic control. The resulting 1,2-dihydropyridines showed resistance to further aromatization. In contrast, furfural consistently yielded 1,4-dihydropyridines regardless of temperature, due to its higher reactivity.
Twelve compounds were synthesized, three of which are novel. Yields ranged from 69 % to 92 %. The process aligns with green chemistry principles, as confirmed by Green Metric indicators such as Atom Economy (AE), E-factor (EF), and Product Mass Intensity (PMI). Sustainability was further visualized through radial pentagon diagrams for representative reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


