在绿色溶剂中多元定向合成氰基取代多环n -杂芳基化合物
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氰化多环n -杂环在制药应用中至关重要;然而,它们的合成往往依赖于不可持续的方法,涉及非绿色溶剂,化学计量化学氧化剂或介质,或贵金属催化剂。在这项研究中,我们报道了一个锰催化的氧化串联反应,以芳基乙酸/芳香醛、杂环胺和三甲基硅基氰化物为原料合成氰化多环n -杂环。该串联反应通过锰催化的streker反应进行,随后是氧化自由基环化和芳构化。值得注意的是,该方案利用了γ-戊内酯作为绿色溶剂,Mn催化剂和γ-戊内酯都可以多次回收。本文章由计算机程序翻译,如有差异,请以英文原文为准。
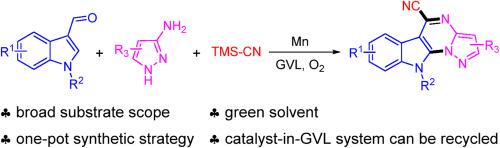
Diversity oriented synthesis of cyano-substituted polycyclic N-heteroaryl compounds in green solvent
Cyanated polycyclic N-heterocycles are vital for pharmaceutical applications; however, their synthesis often relies on unsustainable methods involving non-green solvents, stoichiometric chemical oxidants or mediators, or precious-metal catalysts. In this study, we report a manganese-catalyzed oxidative tandem reaction for synthesizing cyanated polycyclic N-heterocycles from arylacetic acids/aromatic aldehydes, heterocyclic amines, and trimethylsilyl cyanide. This tandem reaction proceeds via a manganese-catalyzed Strecker reaction, followed by oxidative radical cyclization, and aromatization. Notably, this protocol utilizes γ-valerolactone as a green solvent, both Mn catalyst and γ-valerolactone can be recycled multiple times.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


