神经肽Y作为神经可塑性、神经炎症和HPA轴失调的多方面调节剂:对治疗抵抗性抑郁症的感知
IF 2.7
3区 医学
Q3 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
难治性抑郁症(TRD)是一种严重的神经生物学和临床类别,其中常见的抗抑郁药物不能诱导足够的反应。神经肽Y (NPY)是一种在进化过程中高度保守的神经肽,参与调节应激反应、情绪加工、神经炎症和神经可塑性。本文通过描述NPY的生理(中枢神经系统)功能和作用,综合了NPY在TRD中的重要性。实验数据表明,慢性应激和TRD动物模型中NPY失调,包括海马NPY信号、神经营养因子表达和HPA轴活性的改变。反过来,临床研究表明,TRD患者的脑脊液中NPY浓度降低,大脑应激相关区域中NPY受体表达减少。最后,我们讨论了针对NPY病理的新治疗途径,如鼻内NPY给药和受体选择性调节。因此,临床前和临床数据共同表明,恢复NPY信号通路可能为TRD提供一种新颖的、基于生物学的干预措施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
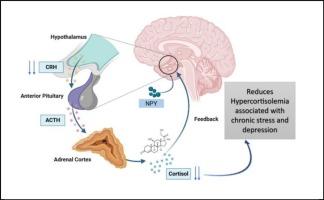
Neuropeptide Y as a multifaceted modulator of neuroplasticity, Neuroinflammation, and HPA axis dysregulation: Perceptions into treatment-resistant depression
Treatment-resistant depression (TRD) is a severe neurobiological and clinical category in which common antidepressants do not induce an adequate response. Neuropeptide Y (NPY), a neuropeptide highly conserved through evolution, is known to be involved in the regulation of stress responses, emotional processing, neuroinflammation, and neuroplasticity. This review synthesizes the importance of NPY in TRD by describing NPY's physiological (central nervous system) functions and roles. Experimental data suggest that NPY is dysregulated in animal models of chronic stress and TRD, which include changes in hippocampal NPY signaling, neurotrophic factor expression, and HPA axis activity. Clinical studies have, in turn, shown decreased cerebrospinal NPY concentrations in TRD patients as well as decreased expression of NPY receptors in stress-related regions of their brains. Finally, we discuss new therapeutic avenues targeting NPY pathology, such as intranasal NPY administration and receptor-selective modulation. Thus, preclinical and clinical data jointly suggest that restoration of NPY signaling pathways might offer a novel and biologically grounded intervention for TRD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropeptides
医学-内分泌学与代谢
CiteScore
5.40
自引率
6.90%
发文量
55
审稿时长
>12 weeks
期刊介绍:
The aim of Neuropeptides is the rapid publication of original research and review articles, dealing with the structure, distribution, actions and functions of peptides in the central and peripheral nervous systems. The explosion of research activity in this field has led to the identification of numerous naturally occurring endogenous peptides which act as neurotransmitters, neuromodulators, or trophic factors, to mediate nervous system functions. Increasing numbers of non-peptide ligands of neuropeptide receptors have been developed, which act as agonists or antagonists in peptidergic systems.
The journal provides a unique opportunity of integrating the many disciplines involved in all neuropeptide research. The journal publishes articles on all aspects of the neuropeptide field, with particular emphasis on gene regulation of peptide expression, peptide receptor subtypes, transgenic and knockout mice with mutations in genes for neuropeptides and peptide receptors, neuroanatomy, physiology, behaviour, neurotrophic factors, preclinical drug evaluation, clinical studies, and clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


