吡虫啉环境浓度对非洲爪蟾肾脏系统的影响:从组织病理学到分子生物学的多方面见解
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
鉴于吡虫啉在水生环境中广泛存在,且其对两栖动物肾脏健康的影响研究有限,本研究探讨了这种常用的新烟碱类杀虫剂对非洲爪蟾肾脏功能的影响及其分子机制。采用28天暴露模型,研究了两种浓度吡虫啉诱导的组织病理学变化和酶反应,以及与环境相关水平的基因表达改变和代谢破坏。结果显示,肾脏组织病理损伤显著,参与氧化应激和神经毒性的关键酶,如超氧化物歧化酶、谷胱甘肽s -转移酶和乙酰胆碱酯酶发生变化。转录组学和代谢组学分析阐明了基因表达和代谢谱的深刻变化,特别是影响碳水化合物、氨基酸和嘌呤代谢途径。这项研究证明了代谢适应的双重作用——既具有保护功能,又可能在持续暴露下导致长期有害影响。这些发现强调需要谨慎管理新烟碱的使用,以减轻对水生野生动物,特别是两栖动物的环境影响,并为保护策略提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
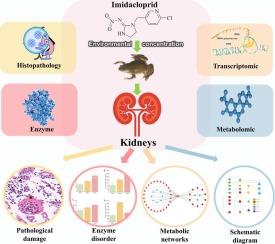
Environmental concentration effects of imidacloprid on the renal system of Xenopus laevis: Multifaceted insights from histopathology to molecular biology
Given the widespread presence of imidacloprid in aquatic environments and the limited research on its impact on amphibian renal health, in this study, we investigated the effects of this commonly used neonicotinoid insecticide on kidney function and molecular mechanisms in Xenopus laevis. Employing a 28-day exposure model, histopathological changes and enzymatic responses induced by two concentrations of imidacloprid were examined, along with gene expression alterations and metabolic disruptions at environmentally relevant levels. The results highlighted significant renal histopathological damage and changes in key enzymes involved in oxidative stress and neurotoxicity, such as superoxide dismutase, glutathione S-transferase, and acetylcholinesterase. Transcriptomic and metabolomic analyses elucidated profound alterations in gene expression and metabolic profiles, particularly affecting carbohydrate, amino acid, and purine metabolism pathways.
This study demonstrates the dual role of metabolic adaptations—serving both protective functions and potentially leading to long-term detrimental effects under continuous exposure. These findings underscore the need for cautious management of neonicotinoid usage to mitigate environmental impacts on aquatic wildlife, particularly amphibians, and inform conservation strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


