从废物到资源:将纺织废料转化为多孔碳吸附剂,用于铵的吸附
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
水产养殖废水中的氨污染对水质和水生生态系统构成严重威胁。传统的氨去除方法成本高、效率低,因此需要更可持续、更经济的替代方法。在本研究中,将消费后的纺织废料在150 °C预氧化,400 °C有限空气中热解,稀释氢氧化钠(NaOH)溶液(0.05 M)改性的温和条件下,通过简单的工艺转化为碳吸附剂。该方法使合成的碳吸附剂具有良好的结构性能和表面化学性质。最佳吸附剂的最大吸附容量为25.3 mg g−1,吸附动力学快速,主要通过静电吸附和离子交换,在5 min内达到90% %的平衡吸附容量。用0.1 M氯化钠(NaCl)溶液对废吸附剂进行再生处理,循环5次后吸附量仅下降9 %。本研究为利用低成本的碳吸附剂处理废水提供了一种潜在的解决方案,并有助于循环经济和可持续发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
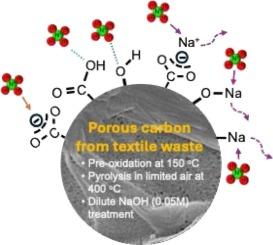
From waste to resource: repurposing textile waste to porous carbon adsorbent for ammonium adsorption
Ammonia pollution in wastewater from aquaculture poses a significant threat to water quality and aquatic ecosystems. Conventional ammonia removal methods can be costly and inefficient, driving the need for more sustainable and affordable alternatives. In this study, post-consumer textile waste was converted to carbon adsorbent via a facile process under mild conditions involving pre-oxidation at 150 °C, pyrolysis at 400 °C in limited air, and modification with dilute sodium hydroxide (NaOH) solution (0.05 M). The combined approach leads to favorable textural properties and surface chemistry of the resultant carbon adsorbent for ammonium adsorption. The optimal adsorbent features a maximum adsorption capacity of 25.3 mg g−1, and fast adsorption kinetics with 90 % of equilibrium adsorption capacity achieved within 5 min, mainly via electrostatic adsorption and ion exchange. The spent adsorbent can be easily regenerated using 0.1 M sodium chloride (NaCl) solution, with the adsorption capacity dropped only 9 % performance after five cycles. This study offers a potential solution for wastewater treatment by using low-cost carbon adsorbents converted from waste materials, and contributes to circular economy and sustainability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


