EPLINα控制Rab21内体的整合素循环,驱动乳腺癌细胞迁移
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肿瘤上皮蛋白丢失(EPLIN)是一种肌动蛋白结合蛋白,在不同的癌症中被描述为肿瘤启动子和肿瘤抑制子。EPLIN异构体(α/β)的作用在很大程度上仍然未知,可以解释这些相反的观点。我们在乳腺癌细胞中观察到明显的EPLIN异构体定位;EPLINα被募集到质膜褶边和核内体的肌动蛋白上,而EPLINβ则位于应力纤维上。EPLINα以动作蛋白依赖的方式定位于早期核内体,在那里它与Rab21相互作用,Rab21是β1-整合素内体运输的既定调节剂。这支持β1-整合素循环和细胞迁移。通过邻近生物素化(BioID),我们确定了冠状蛋白1C是eplin近端蛋白,它也定位于含有rab21的内体,并控制EPLINα下游的整合素循环。EPLINα的表达与乳腺癌细胞运动性增加有关,而患者样本中较高的EPLINα与eplin β比值与间充质表型相关。我们的工作确定了以前未知的与乳腺癌相关的eplin亚型特异性功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
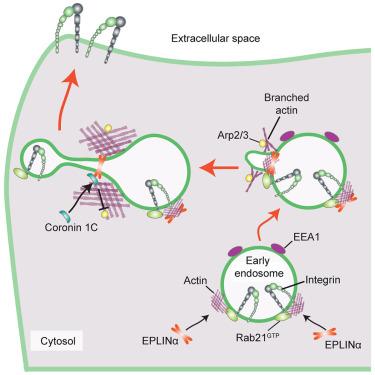
EPLINα controls integrin recycling from Rab21 endosomes to drive breast cancer cell migration
Epithelial protein lost in neoplasm (EPLIN), an actin-binding protein, has been described as both a tumor promoter and tumor suppressor in different cancers. The roles of EPLIN isoforms (α/β) remain largely unknown and could explain these opposing views. We observed distinct EPLIN isoform localization in breast cancer cells; EPLINα is recruited to actin in plasma membrane ruffles and endosomes, while EPLINβ resides on stress fibers. EPLINα localizes to early endosomes in an actin-dependent manner, where it interacts with Rab21, an established regulator of β1-integrin endosomal trafficking. This supports β1-integrin recycling and cell migration. Using proximity biotinylation (BioID), we identified coronin 1C as an EPLIN-proximal protein, which also localizes at Rab21-containing endosomes and controls integrin recycling downstream of EPLINα. EPLINα expression was linked to increased breast cancer cell motility, and a high EPLINα-to-EPLINβ ratio correlated with a mesenchymal phenotype in patient samples. Our work identifies previously unknown EPLIN-isoform-specific functions relevant to breast cancer and beyond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


