自由基s -腺苷-l-蛋氨酸FeS簇在白霉素生物合成过程中作为硫供体
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
天然产物生物合成中的碳-硫键形成反应主要涉及路易斯酸/碱化学,由s -腺苷-l-蛋氨酸(SAM)自由基催化的例子相对较少。后者仅限于自由基介导的硫插入到碳-氢键中,硫原子来自于牺牲的辅助铁-硫簇。本研究表明,在白霉素生物合成基因簇中编码的自由基SAM酶AbmM催化硫氧交换反应,将胞苷5 ' -二磷酸的呋喃糖环转化为硫代呋喃糖,这对白霉素δ2的抗菌活性至关重要。因此,除了具有介导SAM的还原裂解的典型功能外,AbmM的自由基SAM催化簇似乎还在提供AbmM催化反应过程中引入的硫方面发挥作用。这些发现不仅确定了albomycin δ2中硫代铀糖核的起源,更重要的是强调了自由基SAM催化功能的多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
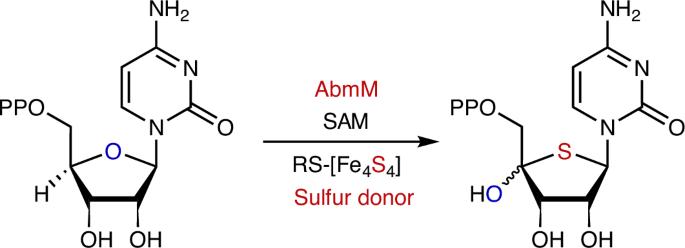
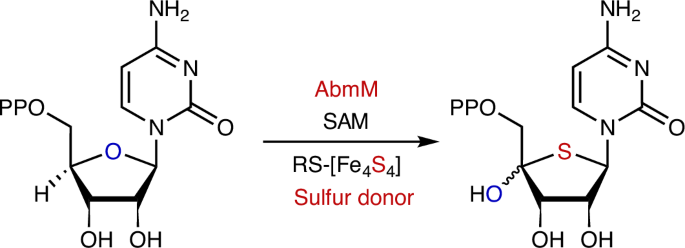
Radical S-adenosyl-l-methionine FeS cluster implicated as the sulfur donor during albomycin biosynthesis
Carbon–sulfur bond-forming reactions in natural product biosynthesis largely involve Lewis acid/base chemistry with relatively few examples catalysed by radical S-adenosyl-l-methionine (SAM) enzymes. The latter have been limited to radical-mediated sulfur insertion into carbon–hydrogen bonds with the sulfur atom originating from a sacrificial auxiliary iron–sulfur cluster. Here we show that the radical SAM enzyme AbmM encoded in the albomycin biosynthetic gene cluster catalyses a sulfur-for-oxygen swapping reaction, transforming the furanose ring of cytidine 5′-diphosphate to a thiofuranose moiety that is essential for the antibacterial activity of albomycin δ2. Thus, in addition to its canonical function of mediating the reductive cleavage of SAM, the radical SAM catalytic cluster of AbmM appears to play a role in providing the sulfur introduced during the AbmM-catalysed reaction. These discoveries not only establish the origin of the thiofuranose core in albomycin δ2 but, more importantly, also emphasize the functional diversity of radical SAM catalysis. The mechanism by which sulfur is incorporated into the furanose ring in albomycins—a group of natural nucleoside antibiotics—remains unclear. Now, this work explains how twitch radical S-adenosyl-l-methionine enzyme AbmM catalyses sulfur-for-oxygen replacement in the biosynthesis of albomycin δ2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


