迁移的免疫细胞全局协调突出力
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
有效的免疫反应依赖于白细胞穿越各种复杂组织的能力。为了适应这种不断变化的环境条件,白细胞通常采用变形虫结构,利用其前向的细胞核作为探针,在已有的孔隙中识别并遵循阻力最小的路径。我们表明,在密集的环境中,即使是最大的孔隙也会阻碍自由通过,白细胞将其细胞核定位在中心体和细胞器的后面。周围环境对细胞体施加的局部压缩触发了位于细胞壁和细胞核之间的中央f -肌动蛋白池的组装。中央肌动蛋白向外推动,暂时扩张细胞器和细胞核的通道。中央和前部的肌动蛋白池是紧密耦合的,中央池的实验性耗竭增强了细胞前部肌动蛋白的积累和突起的形成。虽然这种转移的平衡加速了细胞在宽松环境中的迁移,但在限制性环境中的迁移受到损害,因为释放的前缘与被困的细胞体分离。我们的研究结果建立了一个肌动蛋白调节环,平衡路径扩张和前沿的进步,以保持细胞的一致性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
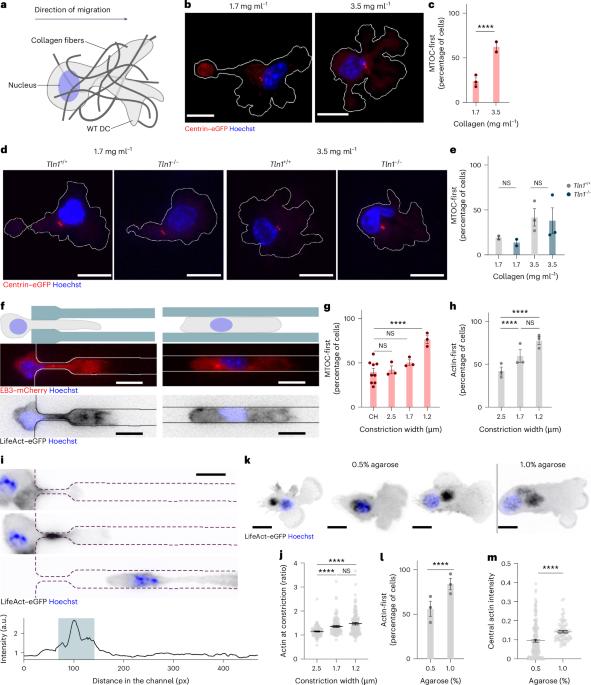

Migrating immune cells globally coordinate protrusive forces
Efficient immune responses rely on the capacity of leukocytes to traverse diverse and complex tissues. To meet such changing environmental conditions, leukocytes usually adopt an ameboid configuration, using their forward-positioned nucleus as a probe to identify and follow the path of least resistance among pre-existing pores. We show that, in dense environments where even the largest pores preclude free passage, leukocytes position their nucleus behind the centrosome and organelles. The local compression imposed on the cell body by its surroundings triggers assembly of a central F-actin pool, located between cell front and nucleus. Central actin pushes outward to transiently dilate a path for organelles and nucleus. Pools of central and front actin are tightly coupled and experimental depletion of the central pool enhances actin accumulation and protrusion formation at the cell front. Although this shifted balance speeds up cells in permissive environments, migration in restrictive environments is impaired, as the unleashed leading edge dissociates from the trapped cell body. Our findings establish an actin regulatory loop that balances path dilation with advancement of the leading edge to maintain cellular coherence. Sixt and colleagues show that, in environments where even the largest pores preclude free passage, leukocytes position their nucleus behind the centrosome and assemble a central F-actin pool that pushes outward to transiently dilate a path for the nucleus.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


