采用d -最优混合设计的格列美脲原位微乳-硅,体外和体内研究。
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Nanomedicine : nanotechnology, biology, and medicine
Pub Date : 2025-07-12
DOI:10.1016/j.nano.2025.102842
引用次数: 0
摘要
目的:格列美脲是一种BCS II类口服降糖药,溶出率有限,吸收有限。口服原位微乳剂可以提高格列美脲的溶解度,通过提高溶出率加快吸收,随后快速起效,减少口服生物利用度的变化,从而有效控制血糖水平。方法:以癸醇90、Tween 20、PEG 400和硬脂酰胺为原料,采用D-Optimal混合设计制备格列美脲原位微乳。在硅分子模拟研究进行了解硬脂酰胺在改变格列美脲溶解度的作用。对原位微乳进行了粒径、zeta电位、多分散性指数、药物含量、pH值和加速老化等表征。进一步,优化后的原位微乳通过吸附在Syloid XDP 3150上转化为颗粒。结果:分子动力学模拟证实硬脂酰胺增强格列美脲在Capryol 90中的溶解度。DSC热像图显示格列美脲的无定形状态,而体外释放研究表明,在10 min结束时,格列美脲从原位微乳和颗粒中释放,与悬浮液相比,释放量为bbb90 %。对Wistar大鼠未外翻的肠囊进行的离体研究显示,格列美脲颗粒的离体肠通透性比悬浮液高。在雄性Wistar大鼠体内的研究表明,开发的格列美脲颗粒具有统计学意义(p )。结论:本研究工作具有原位递送格列美脲微型乳剂有效治疗糖尿病的潜力,并为提高口服生物利用度提供了新颖性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
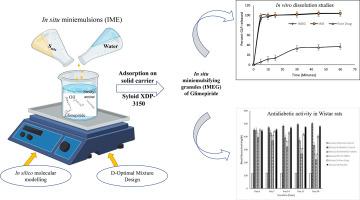
In situ miniemulsions of glimepiride using D-optimal mixture design-in silico, in vitro and in vivo studies
Purpose
Glimepiride, a BCS class II oral hypoglycaemic drug shows dissolution rate limited absorption. Oral in situ miniemulsions can improve Glimepiride solubility by enhancing dissolution rate for faster absorption, subsequently rapid onset of action and reducing variations in oral bioavailability for effective control of blood glucose levels.
Methods
Capryol 90, Tween 20, PEG 400 and stearyl amine were used to develop in situ miniemulsions of Glimepiride using D-Optimal mixture design. In silico molecular modelling studies were performed to understand role of stearyl amine in modifying Glimepiride solubility. In situ miniemulsions were characterized for globule size, zeta potential, polydispersity index, drug content, pH and accelerated aging. Further, the optimized in situ miniemulsions were converted into granules by adsorbing on Syloid XDP 3150.
Results
Molecular dynamics simulation confirmed that stearyl amine enhanced Glimepiride solubility in Capryol 90. DSC thermograms revealed amorphous state of Glimepiride while in vitro release studies indicated >90 % Glimepiride release from in situ miniemulsions and granules as compared to suspensions at the end of 10 min. Ex vivo studies performed on non-everted gut sacs from Wistar rats displayed greater ex vivo intestinal permeability of Glimepiride from the developed granules than suspensions. In vivo studies in male Wistar rats revealed that developed Glimepiride granules showed statistically significant (p < 0.001) decrease in blood glucose levels as compared to conventional marketed tablets of Glimepiride.
Conclusions
The present research work holds potential in delivering in situ miniemulsions of Glimepiride for effective treatment of diabetes and proposes novelty for enhancing oral bioavailability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
0.00%
发文量
133
审稿时长
42 days
期刊介绍:
The mission of Nanomedicine: Nanotechnology, Biology, and Medicine (Nanomedicine: NBM) is to promote the emerging interdisciplinary field of nanomedicine.
Nanomedicine: NBM is an international, peer-reviewed journal presenting novel, significant, and interdisciplinary theoretical and experimental results related to nanoscience and nanotechnology in the life and health sciences. Content includes basic, translational, and clinical research addressing diagnosis, treatment, monitoring, prediction, and prevention of diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


