碱基催化的单糖转化为甲酸的级联:同位素跟踪揭示了在温和条件下在水中的途径及其最佳使用
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
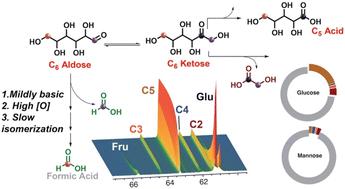
如果开发出操作简单且高产的工艺,碳水化合物是未来前体和燃料的有希望的底物。有吸引力的产品包括甲酸作为一种安全处理的液相储氢材料。甲酸盐可以通过丰富的单糖如葡萄糖在双氧水的碱性溶液中转化而形成。这种转换的细节和最佳使用策略仍然难以捉摸。在这里,我们展示了实时同位素跟踪和定量核磁共振为葡萄糖转化为甲酸及其副产物的复杂途径提供了见解。与之前的看法相反,果糖并不是这些反应的副产品。相反,单糖的降解和产物组成是由竞争控制的(i) C-C键在羰基附近的裂解和(ii)通过Lobry de Bruyn-Van Ekenstein转化进行异构化。与葡萄糖中的质子相比,过氧化氢的酸性更强,这有助于葡萄糖在中等碱性条件下转化为甲酸盐。具有中等碱度的替代介质,如硅酸盐和磷酸盐代替氢氧化物,可以提供有效的葡萄糖转化。相比之下,高碱性环境和氧化剂浓度不足会增加葡萄糖异构化成果糖,果糖迅速转化为副产品C2-C5醛酸。高氧化剂浓度和底物结构确保了低醛糖到酮糖异构化率,有利于甲酸形成,比C2-C5醛醛酸形成的比例超过20:1。总的来说,深入了解降解级联中的竞争途径,提出了在动力学控制下优化普通醛糖转化为甲酸盐的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Base-catalyzed cascades of monosaccharide conversion to formic acid: isotope tracking reveals pathways and their optimal usage under mild conditions in water†
Carbohydrates are promising substrates for future precursors and fuels if operationally simple and high-yielding processes are developed. Attractive products include formic acid as a liquid-phase hydrogen-storage material that is safe to handle. Formate can be formed through the conversion of abundant monosaccharides such as glucose in aqueous alkaline solution of hydrogen peroxide. Details of this conversion and strategies for its optimal usage have remained elusive. Here, we show that real-time isotope tracking and quantitative NMR provide insights into the complex pathways of glucose conversion to formate and its byproducts. Contrary to previous belief, fructose is not a byproduct of these reactions. Instead, monosaccharides degradation and product composition is governed by competing (i) C–C bond cleavage adjacent to carbonyl groups and (ii) isomerization via the Lobry de Bruyn–Van Ekenstein transformation. The stronger acidity of hydrogen peroxide compared to protons in glucose is found to support glucose conversion to formate under moderately basic conditions. Alternative media with moderate basicity, such as silicate and phosphate salts instead of hydroxides, can provide efficient glucose conversion. By contrast, highly alkaline environments and insufficient oxidant concentrations increase glucose isomerization to fructose, which rapidly converts to C2–C5 aldonic acids as byproducts. High oxidant concentration and substrate configurations that ensure low aldose-to-ketose isomerization rates favor the formation of formate over C2–C5 aldonic acids by more than 20 : 1. Overall, insight into the competing pathways in the degradation cascade suggests strategies for optimizing the conversion of common aldoses to formate under kinetic control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


